CSS Forums
Saturday, April 20, 2024
01:45 AM (GMT +5)
01:45 AM (GMT +5)
|
#1
|
||||
|
||||
|
General Science This thread contains all info regarding `General Science` and all related facets of science. Topic # 1 MATTER AND MATERIALS 1. MATTER Everything you can hold, taste, or smell is made of matter. Matter makes up everything you can see, including clothes, water, food, plants, and animals. It even makes up some things you cannot see, such as air or the smell of perfume. You can describe a type of matter by its MATERIAL PROPERTIES such as its colour or how hard it is. Matter is made up of PARTICLES so tiny that only the most powerful microscope can see them.  NON-MATTER Not everything is made of matter. Non-matter includes the light from a torch, the heat from a fire, and the sound of a police siren. You cannot hold, taste, or smell these things. They are not types of matter, but forms of energy. Everything that exists can be classed as either a type of matter or a form of energy. MATERIAL PROPERTIES Different types of matter have different material properties that make them useful for different jobs. A plastic hosepipe is flexible, so it can be pointed in any direction. A perspex visor is transparent, so the wearer can see straight through it. A firefighter’s suit is shiny so it can reflect heat and light. Flexibility, transparency, and shininess are three examples of material properties. PARTICLES All matter is made of incredibly tiny particles called atoms. Atoms are far too small to see with our eyes, but scientists have worked out how small they are. There are many kinds of atom. Sand grains are made of two kinds of atom: oxygen and silicon. People are made of about 28 different kinds of atom. Material properties depend on the kinds of atom the material is made from. SAND PARTICLES Grains of sand look like pieces of gravel when viewed through a microscope. They have different shapes and sizes. Each grain contains millions of atoms, too small to see with a microscope. A sand grain the size of the full stop at the end of this sentence would contain about 10 million million million atoms. ATOMS We cannot really see atoms with microscopes. The best we can do is image them, by bouncing light off the particles. A computer translates the light beams into an image. Scanning tunnelling microscopes (STM) and atomic force microscopes (AFM) do this. BIOGRAPHY: DEMOCRITUS Greek, 460-c. 370 BC Democritus was one of the first philosophers (thinkers) to say that everything was made up of particles too small to be seen. He believed these particles could not be destroyed or split. Democritus said that all changes in the world could be explained as changes in the way particles are packed together. 2. SOLIDS Solids are one of the three states of matter and, unlike liquids or gases, they have a definite shape that is not easy to change. Different solids have particular properties such as stretch, STRENGTH, or hardness that make them useful for different jobs. Most solids are made up of tiny crystals. This is because their particles are arranged in a regular pattern, called a CRYSTALLINE STRUCTURE.  CHANGING SHAPE Some solids can be hammered or squashed into many different shapes without breaking. They are known as malleable materials. Other solids, such as biscuits or glass, will not bend when hammered or squashed, but will break and split. These materials are brittle. SHAPE MEMORY METAL Shape memory metals can remember their shape. When brought to a certain temperature, these metals can be set to a shape that they never forget. They have many uses, including repairing broken bones. Even if the bones move, the metal always returns to its original shape, bringing the bones back to their correct position. Stretch Some solids, such as the metal copper, can be pulled and stretched easily into extremely thin wires. They are known as ductile materials. They have this property because their particles are not held in a rigid structure, but are arranged in rows that can slide past one another. Copper can be stretched into a thread half the width of a human hair, and is used in many kinds of wiring, including electrical and telephone wiring. STRENGTH Some solids, such as steel or concrete, are difficult to break, even if they are made to carry a heavy weight. This is because their particles are bound together very strongly. Such materials are said to have high strength and are used to construct bridges and buildings. Strength is a different property from hardness. Hard materials cannot be bent or scratched easily. MOHS HARDNESS SCALE Hardness is a measure of how easily a material can be scratched. Mohs hardness scale arranges 10 minerals from 1 to 10. The higher the number, the harder the mineral. Each mineral in the scale will scratch all those below it. Other materials can be compared to these minerals. Copper, for example, has a hardness of 2.5. CRYSTALLINE STRUCTURE Most solids, such as metals, salt, and sugar, are made up of tiny crystals. Their particles are arranged in regular three-dimensional patterns such as cubes or hexagonal shapes. Not all solids are like this, however. The particles of glass, for example, are not arranged in a regular pattern, and so glass does not have a crystalline structure. Its structure is described as amorphous. 3. LIQUIDS As water flows along a river, it constantly changes its shape to fit the space available. This is because water is a liquid, and liquids flow and do not have a fixed shape. Instead, they take on the shape of whatever container they are in. If you pour a liquid from a glass onto a plate, the volume of liquid (the space it takes up) stays the same, but its shape changes.  COHESION Mercury is a liquid metal that is poisonous. When mercury is dropped onto a surface, it rolls off in little balls. This is because the forces between the mercury particles are very strong, so the particles clump together. This force between particles of the same type is called cohesion. Water particles do not have such strong cohesion, so they wet surfaces. VISCOSITY A measure of how fast or slowly a liquid can flow is its viscosity. Crude oil, for example, is a liquid that does not flow very easily. It is said to have high viscosity. Heating crude oil lowers its viscosity and enables it to flow more freely through pipes. Other liquids, such as water, flow easily without being heated. Water has low viscosity. VOLUME Although they look very different, these two containers contain the same volume of liquid. The volume of a liquid is the amount of space it takes up. Although liquids change their shape when moved from one container to another, their volume always stays the same. For this reason, liquids are usually measured by their volume, in litres or gallons. 4. GASES Gases are all around us, but although many, such as perfume, can be smelt, most gases are invisible. Like liquids, gases can flow but, unlike solids or liquids, gases will not stay where they are put. They have no set shape or volume, and they expand in every direction to fill completely whatever container they are put into. If the container has no lid, the gas escapes.  EXPANSION In hot air balloons, a burner heats the air inside. This causes the particles of air to gain more energy and so they move faster and farther apart from one another, pushing at the sides of the balloon. Heat always causes gases to expand. If you left a balloon near a fire, the air inside could expand so much that the balloon would burst. 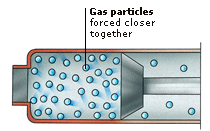 PRESSURE Why does a champagne cork explode out of a shaken bottle? The champagne inside the bottle contains lots of tiny bubbles of gas. Shaking the bottle releases the gas, and the high-speed gas particles bang against the cork. This creates an enormous pressure on the cork, and eventually forces the cork out of the bottle. 5. MIXTURES Almost everything is made of different substances mixed together. Things are only easy to recognize as mixtures if the PARTICLE SIZE of each substance is big enough to see. The flakes, nuts, and raisins in a bowl of cereal are a mixture that is easy to see. A fruit drink, though, doesn’t look like a mixture because the particles of fruit and water are so small. It is a type of mixture called a SOLUTION, made of different, very tiny particles dissolved (evenly spread out) in water. COMMON PARTICLES Rock, sand, and seawater are all mixtures of the same substances – such as the minerals feldspar, mica, and quartz – but in different particle sizes. Rock contains these substances in chunks or veins; sand has them as small grains; and seawater contains them as tiny dissolved particles that are invisible to the eye. Rain and rivers dissolve the minerals as they wash over the rock on their way to the sea. MINERAL MIXTURES All rocks are mixtures of naturally occurring substances called minerals. Granite is a common rock made of three differently coloured minerals called feldspar, mica, and quartz. The pink grains in granite are feldspar, the black grains are mica, and the light grey, glass-like grains are quartz. Granite is usually about 75% feldspar, 5% mica, and 20% quartz. These proportions can vary and the rock often contains small amounts of other minerals as well.  PARTICLE SIZE There are many different types of mixtures, which are divided into groups based on how small their particles are. A mixture such as sand has a large particle size. Mud stirred in water is a type of mixture called a suspension; the particles are too small to see when mixed, but they eventually settle out. A mixture such as fog (water and air) is called a colloid; its particles are too small ever to settle out. COARSE MIXTURES The particles of some mixtures are large enough to see without a microscope. When you look closely at a handful of sand, for example, you can make out the different coloured grains mixed together. Some sands have smaller grains than others. The smaller the grain size, the softer and more powdery the sand feels. COLLOID A colloid is a mixture containing tiny particles of one substance scattered throughout another substance, such as dye particles mixed with glass in a marble. The particles are smaller than those in a suspension, but larger than those in a solution. The particles are so small and light, they do not ever settle out. EMULSION Milk is made up of tiny globules of fat scattered throughout water. It is an example of an emulsion, a special type of colloid in which oils or fats are mixed with water to create a creamy liquid or paste. Other examples of emulsions are mayonnaise, emulsion paints, lipsticks, and face creams. SOLUTIONS A solution is a mixture in which the different particles are tiny and are mixed completely evenly. Solutions are often made by dissolving a solid, such as sugar, into a liquid, such as water. The sugar is called the solute and the water is called the solvent. Water is the most common solute. Solutions can also be a liquid dissolved in another liquid, for example antiseptic liquid. This is water and alcohol. Or they can be a gas dissolved in another gas, such as oxygen dissolved in nitrogen in the air. SOLID SOLUTION Wood’s Metal is found in automatic fire sprinklers. It is an alloy (mixture of metals) containing bismuth, lead, tin, and cadmium. This mix of metals has a low melting point of 71ºC (158ºF). It is used as a sensor in automatic fire sprinklers; if the temperature gets too high, the metal alloy melts and releases the water. 6. ELEMENTS  The enormous variety of matter around you is made from different combinations of substances called elements. Elements are pure substances that cannot be broken down into anything simpler. Some, such as gold and silver, are found on their own. Most elements, however, are combined in twos, threes, and more to make compounds. The NATURAL ELEMENTS are found on Earth. SYNTHETIC ELEMENTS are created in laboratories and are often short-lived. METEORITE An element is always the same, wherever it is found. For example, meteorites are large rocks that have landed on Earth from space. Some meteorites contain metal, such as iron, which is a natural element. The iron in a meteorite from space is exactly the same as iron found in rocks on Earth. ORIGIN OF ELEMENTS All the elements on Earth were formed in the heart of exploding stars. The early Universe was made of just two elements, hydrogen and helium, which formed into stars. At the fiery core of these stars, the hydrogen and helium were forced together to form new, heavier elements. Even heavier elements were created in the explosions of massive stars, called supernovas. Table 2. ELEMENTS IN THE EARTH'S CRUST ELEMENT PERCENTAGE Oxygen 47 Silicon 28 Aluminium 8 Iron 5 Calcium 3.5 Sodium 3 Potassium 2.5 Magnesium 2 All other elements 1 BIOGRAPHY: JOHN DALTON English, 1766–1844 Chemist John Dalton studied the gases in air. He proposed that everything was made from simple substances called elements. He said that the properties (characteristics) of every particle of one element are identical, and are different to the properties of any other element. This is how elements are defined today. NATURAL ELEMENTS There are 90 natural elements, ranging from the lightest, hydrogen, to the heaviest, uranium. Other familiar elements are aluminium, carbon, copper, and oxygen. Every substance on Earth is made up of one or more of these 90 elements. Oxygen is the most common element on Earth. Hydrogen is the most common element in the Universe. ALUMINIUM Aluminium is a common element, but it is never found naturally on its own. It has to be extracted from rocks called minerals. This extraction process used to be very difficult and aluminium was once considered a precious metal, more valuable than gold. Nowadays, extraction is much easier, and aluminium is used for many everyday items, such as drinks cans and foil. SYNTHETIC ELEMENTS No element heavier than uranium is found naturally. Scientists can, however, collide two smaller elements together at high speeds to form a new, heavier element. Many elements made this way break apart almost immediately, although a few can stay together for a few days or even weeks. Scientists make them to learn more about how elements form and how they change as they get heavier. Synthetic elements include plutonium and einsteinium. CYCLOTRON Scientists create synthetic elements in a cyclotron. The cyclotron contains a circular track, into which particles are released. The particles are speeded up to extremely high speeds, before being allowed to collide with a target of another element to form new elements. In the largest cyclotrons, the ring is many kilometres wide and the particles speed at 225,000 kph (13,809 mph). 7. ATOMS 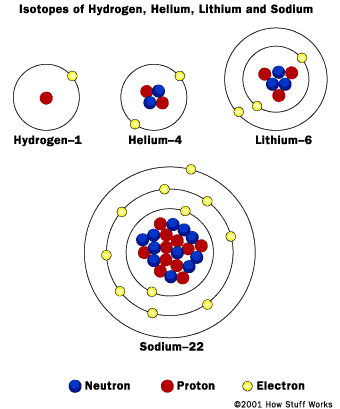 An atom is the smallest part of an element that can exist on its own. Copper, for example, is made from copper atoms, which are different to the oxygen atoms that make up oxygen. Atoms are so tiny that even the full stop at the end of this sentence has a width of around 20 million atoms. Inside each atom are even smaller particles, called subatomic particles. These include a nucleus, which contains protons and neutrons, and electrons that whizz around the nucleus. FOOTBALL STADIUM Imagine an atom magnified to the size of a football stadium. The nucleus of the atom would be the size of a pea in the centre of the stadium, and the electrons would be whizzing around the outer stands. Everything in between would be empty space. NUCLEUS The nucleus is a tightly bound cluster of protons and neutrons. This carbon atom nucleus has 6 protons and 6 neutrons. Protons have a positive electric charge and neutrons nave no charge. Positively charged protons would normally repel each other, but the nucleus is held together by a powerful force called the strong nuclear force. ATOMIC NUMBER Every element has a different atomic number, depending on the number of protons its atoms have in their nuclei. A carbon atom, for example, has 6 protons in its nucleus and so carbon has an atomic number of 6. If the number of protons in the nucleus changes, the atom becomes a completely different element with different properties (characteristics). ATOMIC MASS Atoms of different elements vary in mass. Their mass depends on the number of protons and neutrons in their nucleus. A hydrogen atom has one proton and no neutrons, so it has an atomic mass of one. The greater the atomic mass of an atom, the smaller the atom is. ELECTRIC CHARGES An atom is usually electrically neutral, which means that it has exactly the same number of positively charged protons as it does negatively charged electrons. In this way, the charges cancel one another out. A carbon atom, for example, always has 6 protons and 6 electrons, and usually has 6 neutrons (although different carbon atoms may contain slightly different numbers of neutrons). BIOGRAPHY: NIELS BOHR Danish, 1885-1962 In 1913, Bohr published his model of atomic structure in which electrons travelled in orbits around the central nucleus. He also introduced the idea of electron shells, saying that the properties of an atom depended on how its electrons were arranged in the shells. In 1922, Bohr was awarded the Nobel Prize for Physics. to be continued
|
| The Following User Says Thank You to Sureshlasi For This Useful Post: | ||
Faryal Shah (Saturday, August 02, 2008) | ||
|
#2
|
||||
|
||||
|
8. PERIODIC TABLE
At first glance, the periodic table looks very complex. In fact it is a large grid of every element that exists. The elements are arranged in order of their atomic number. The atomic number is the number of protons each atom has in its nucleus. By arranging the elements in this way, those with similar properties (characteristics) are grouped together. As with any grid, the periodic table has rows running left to right, and columns running up and down. The rows are called PERIODS and the columns are called GROUPS.  Hydrogen (H) is the first element in the periodic table because it has just one proton in its nucleus. Helium (He) is second, because it has two protons, and so on. The periodic table can be coloured-coded. Often, each group is given a particular colour so that it is easy to pick out all the elements that belong to a particular group.  As well as a name, each element has a symbol, a shorthand way of writing the element in chemical equations. Often this is the first letter or two of the element’s name, but it can come from a Latin name. Each also has an atomic number and a mass number. GALLIUM One element that Mendeleyev left a gap for in his periodic table was gallium (element 31). Mendeleyev called it eka-aluminium because he predicted it would have similar properties to aluminium. In 1875, French scientist Lecoq de Boisbaudran discovered gallium. It has the exact properties that Mendeleyev predicted. Gallium is a soft, silvery metal with a melting point of 29.8ºC (85.6ºF). BIOGRAPHY: DIMITRI MENDELEYEV Russian, 1834-1907 This chemist was convinced there was an order to the elements. He collected information on each one and, in 1869, he published a table of elements on which the modern periodic table is based. He left gaps for elements he predicted would be found, such as gallium, germanium, and scandium. GROUPS There are 18 groups (columns) in the periodic table. Group 1 (also known as the alkali metals) is the column on the far left of the table. Elements in the same group have similar, but not identical characteristics. This is because they all have the same number of electrons in their outermost shell. You can tell a lot about an element just by knowing which group it is in. INCREASING SIZE As you move down one element in a group, there is a large jump in the number of protons and neutrons in the nucleus, and a new shell of electrons is added. The extra particles make the atom heavier and the extra shell of electrons makes the atom take up more space. METAL IN SPACE An astronaut’s visor is gold-plated to reflect sunlight. This shiny, hard-wearing metal does not corrode (rust), making it ideal for use in space, where materials cannot be replaced easily. Gold, copper, and silver belong to group 11. Group 11 metals are also called coinage metals, because they are used to make coins. PERIODS The properties of the elements across a period (row) change gradually. The first and last elements are very different. The first is a reactive solid – it catches fire when it mixes with oxygen – and the last is an unreactive gas. However, they have the same number of electron shells. All the elements in the third period, for example, have three shells for their electrons. DECREASING SIZE As you go across a period, the atoms get slightly heavier, but they also get smaller. This is because the number of electron shells stays the same across the period, but the number of protons in the nucleus increases. The stronger, attractive force from the positively charged protons sucks the negatively charged electrons tighter into the centre. PHOSPHORUS MATCH Phosphorus is a non-metal element. It is a yellowish, waxy, slightly see-through solid. Like magnesium, it is very reactive. Because of this, phosphorus compounds are used on the tips of matches. Phosphorus glows in the dark, an effect called phosphorescence. UNREACTIVE ARGON Argon is very unreactive and does not combine with other elements. In arc welding, metals are melted surrounded by argon gas. The argon keeps oxygen out, so that oxygen cannot react with the melted metals. 9. MOLECULES Most atoms join up with other atoms through chemical BONDS to form larger particles called molecules. They can join up with atoms of the same element or with atoms of different elements. Substances whose molecules contain different types of atom are called compounds. Chemical reactions can CHANGE MOLECULES and when this happens, new molecules and therefore new compounds are formed. VARIETY OF MOLECULES Molecules can be simple or complex. They can even be made up of just one atom. The element argon is a one-atom molecule. Other molecules can consist of two atoms of the same element. The oxygen molecule is made up of two oxygen atoms bonded together. However, in certain circumstances, three oxygen atoms bond together, forming a molecule called ozone. SIMPLE MOLECULE Water molecules (H20) are very simple. They are made of two hydrogen (H) atoms bonded to one oxygen (O) atom. All water molecules are the same, but they are different from the molecules of any other substance. A water molecule is the smallest possible piece of water. You can break it up into smaller pieces, but they wouldn’t be water anymore. The symbols that scientists use to represent molecules are called chemical formulae. COMPLEX MOLECULE Some molecules, such as the plastic in a snorkel, contain hundreds or even thousands of carbon, hydrogen, and chlorine atoms joined together in long, winding chains. Such complex molecules are called polymers. They are possible because carbon atoms are able to form very stable bonds with other carbon atoms. Most of the molecules that make up living things are made of complex polymers. BONDING When atoms join together to form molecules, they are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons between the atoms. It is only the electrons in the outermost shell that ever get involved in bonding. Different atoms use these electrons to form one of three different types of bond: ionic bonds, covalent bonds, or metallic bonds. DIFFERENT KINDS OF BONDS BETWEEN ATOMS IONIC BONDS In ionic bonds, electrons are transferred from one atom to another. When sodium and chlorine combine to form sodium chloride (salt), sodium loses an electron and becomes positively charged; chlorine takes that electron and becomes negatively charged. Ionic bonds are difficult to break. Ionic compounds are usually solids with high melting points. COVALENT BONDS In a covalent bond, electrons are shared between two atoms. When two oxygen atoms bond together to form an oxygen molecule, they share four electrons – two from each oxygen atom. Other examples of covalent bonding are water (H2O), and carbon dioxide (CO2). Covalent compounds are usually liquids or gases with low melting points. METALLIC BONDS Metal atoms are bonded to each other through metallic bonding. In this type of bonding, all the atoms lose electrons, which float around in a common pool. The electrons in this pool can move around freely, which is why metals can transfer heat or electricity so well. If one part of the metal is heated, the electrons carry the heat quickly to other parts. CHANGING MOLECULES All around you, molecules are changing and rearranging their atoms in chemical reactions to form new molecules and new compounds. When you breathe in oxygen, it goes through a chemical change inside your body and forms a new compound, carbon dioxide, which you breathe out. Catalysts are special types of molecules that speed up chemical reactions, but do not actually change themselves. They are used, for example, in catalytic converters in cars. SFX REACTION A special effects explosion is a chemical reaction that releases energy. Pyrotechnic experts want each explosion to be unique, so they use different types and amounts of explosives. In every chemical reaction, some bonds between atoms are broken and new ones are made. Energy is needed to break a bond, but energy is released when a bond is made. Depending on the number and type of bonds broken and made, a reaction may take in or give out energy. CATALYTIC CONVERTER When a car engine burns petrol, it releases harmful gases. Cars fitted with a catalytic converter change the harmful gases into safer gases. When they enter the catalytic converter, the gases form temporary bonds with the surface of the catalyst. This brings them into close contact with each other and allows new, safer gases to form. ENZYMES IN THE KITCHEN Enzymes are catalysts found in nature. For example, it is the enzymes in yeast that cause bread dough to rise. When yeast is mixed with warm water and sugar it starts to grow and bubbles of carbon dioxide gas are produced. When the yeast mixture is added to flour and water to make a dough, the dough rises. Heating bakes the bread and kills the yeast. Scientists use chemical equations to show how molecules change in a chemical reaction. 10. CHEMICAL REACTIONS In a chemical reaction, the molecules of one substance break apart and join together with those of another substance to create a different compound (combination of molecules). Many chemical reactions are NON-REVERSIBLE CHANGES .You cannot turn a baked cake back into its raw ingredients. Some chemical reactions can be reversed, and re-formed into the original substances. These are REVERSIBLE CHANGES. PHYSICAL CHANGE A melting ice lolly is an example of a physical change, not a chemical change. The liquid ice lolly is not a new material, just a different form of the old one. Physical changes do not create new substances and no chemical bonds are broken or made. Melting, freezing, tearing, bending, and crushing are all physical changes that alter a substance’s appearance but not its chemical properties. NON-REVERSIBLE CHANGE Many chemical reactions are non-reversible changes. This means they are permanent changes that cannot be undone. You cannot turn the new materials made back into the original materials again. Rusting is a non-reversible change. However, if rust is mixed with magnesium powder another chemical reaction occurs and iron can be extracted from the rust. BURNING Burning is a non-reversible chemical change. When you burn wood, the carbon in the wood reacts with oxygen in the air to create ash and smoke, and energy in the form of light and heat. This is a permanent change that cannot be undone – you cannot turn ashes back into wood. REVERSIBLE CHANGE A few chemical reactions can be reversed – the original materials can be re-created from the new materials. These reactions are called reversible changes. They have a forward reaction and a backward reaction. Both reactions are actually happening at the same time but, depending on the conditions, one will be stronger than the other. NITROGEN DIOXIDE When the gas nitrogen dioxide is heated, a forward chemical reaction changes the brown nitrogen dioxide gas into two colourless gases – nitrogen monoxide and oxygen. However, if these colourless gases are cooled, they will re-form into brown nitrogen dioxide gas. This is called a backward chemical reaction. 11. ACIDS The sour taste of food such as lemons is due to acids. Acids in food are weak, but they can sting if they touch a cut on your skin. Strong acids, such as sulphuric acid in car batteries, are much more dangerous as they can burn through materials. Acid compounds all contain hydrogen. They dissolve in water to produce particles called hydrogen ions. The more hydrogen ions an acid contains, the stronger an acid it is.  Lemons and other citrus fruits taste sour because they contain citric acid. Citric acid is used to add a tangy taste to food and soft drinks. Citrus fruits also contain another acid, called ascorbic acid or Vitamin C, which we need for healthy skin and gums. 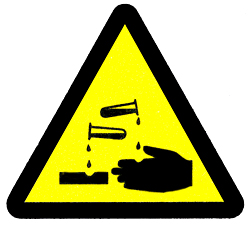 Strong acids and bases are extremely poisonous, corrosive, and cause bad burns, so their containers are labelled with hazard symbols. Some give information about how to handle the chemicals safely. The symbols are also displayed on the tankers that transport acids and bases, so emergency services know how to handle the substances in the case of an accident or spillage. 12. BASES Many cleaning products, such as soap and oven cleaner, are bases. Bases neutralize (cancel out) acids. Alkalis are bases that dissolve in water. Strong bases, such bleach, are corrosive and burn skin. Bases contain particles called hydroxide ions. The more hydroxide ions a base contains, the stronger it is. LIMESTONE Limestone is an important base that is dug from the earth in quarries. It comes from calcium carbonate, which formed millions of years ago from the compressed remains of sea-shells and other marine life. Once quarried, limestone is crushed and used to make cement, fertilizers, paints, and ceramics. 13. METALS Almost three-quarters of all elements are metals, such as gold and silver. There are also some elements we may not think of as metals, such as the calcium in our bones, and the sodium in table salt (sodium chloride). Metals are defined by their METALLIC PROPERTIES, such as high melting points. Mixtures of metals are called ALLOYS. Solder is an alloy that is used to join metals in plumbing and electrical wiring. It is mainly tin with lead or silver. METAL GROUPS Metals are classified according to where they are found in the periodic table. Each group has a set of properties that make the metals useful for different purposes. ALKALI METALS These include potassium and sodium, and form Group I of the periodic table. They are extremely reactive metals: they react strongly with water to form strong alkalis. ALKALINE-EARTH METALS These elements make up Group II of the periodic table. They combine with many elements in the Earth’s crust. Their oxides react with water to form alkalis. TRANSITION METALS This group includes copper, silver, and gold. They are hard and shiny, have high melting points, and are good conductors of heat and electricity. OTHER METALS Also called poor metals, these metals are fairly soft and melt easily. They include tin and aluminium and are often used in alloys. Bronze is an alloy of tin and copper. GOLD IN QUARTZ Some metals, such as gold, are found naturally as pure metals in rocks. Gold is unreactive, so it does not combine with other elements. Most metals are more reactive and are found combined with other elements in rocks. Iron, for example, is usually combined with oxygen. The rocks in which metals are found are called ores. EXTRACTING GOLD To extract gold from its ore, huge grinders crush the ore to a fine powder. The powder is mixed with a solution of cyanide. Only the gold from the ore dissolves in the solution. Powdered zinc is added to bring the gold out of the solution. The gold is melted down and poured into moulds. METALLIC PROPERTIES Metals are usually shiny solids with high melting points and are very good conductors of heat and electricity. They are malleable, so they can be beaten into sheets, and ductile, which means they can be drawn into wires. Most are strong and cannot be broken easily. Of course, there are exceptions: mercury, for example, is a metal that has a low boiling point and is liquid at room temperature. ELECTRICAL CONDUCTORS The transmission lines (electric cables) that bring electricity to our homes, schools, and offices all rely on copper. Copper is a red-orange metal that is one of the best electrical conductors. Metals conduct electricity well because when metal atoms bond (join together), the electrons in their outer shells move freely. If electricity passes through one part of the metal, the electrons carry the electricity quickly to other parts. ALLOYS Alloys are mixtures of metals with properties that make them more useful than pure metals. A mixture of chromium and iron resists rusting much better than iron on its own. Most alloys are made of two or more metals, but some contain a non-metal. Steel is an alloy of iron and carbon. Alloys are made by melting the different materials together. Changing the proportions of the materials can change the properties of the alloy. 14. NON-METAL ELEMENTS The metal elements in the periodic table have easily defined properties. The remaining elements, however, have very different properties. They consist of a group of unreactive gases called the NOBLE GASES, a group of reactive elements known as the HALOGENS, and a set of elements referred to as non-metals. In addition, a few elements have properties that place them in between metals and non-metals. They are called the SEMI-METALS. SULPHUR CRYSTALS Deposits of the non-metal sulphur are found as deep as 300 m (1,000 ft) below ground. Combined with other elements, sulphur is also found in rocks and minerals, such as gypsum. MAKING SULPHURIC ACID Sulphur crystals are ground to a powder at sulphur processing plants. The powder is sprayed into a furnace where it reacts with oxygen, forming sulphur dioxide. More oxygen is reacted with the sulphur dioxide to make sulphur trioxide, which is dissolved in water to make oleum. TRANSPORTING SULPHURIC ACID Oleum is concentrated sulphuric acid. It is transported to manufacturing plants in tankers. Here, water is added to the oleum in precise measures to make the correct concentration of sulphuric acid. Sulphuric acid is used in the manufacture of detergents, paints, medicines, plastics, and synthetic fabrics. SEMI-METALS The elements known as semi-metals have some of the properties of metals and some of the properties of non-metals. Arsenic, for example, has the shininess of a metal but does not conduct heat or electricity very well. Other semi-metals, such as silicon and germanium, are semi-conductors. This means that they can conduct electricity, but only under special conditions. This property makes them very useful in solar panels and computers. HALOGEN At first sight, the halogens don’t seem very alike. For example, fluorine is a yellow gas and iodine is a shiny, black solid. However, they are all highly reactive and are quick to combine with other elements to form salts, such as table salt (sodium chloride). They also have important uses. Chlorine is used to disinfect water, and compounds of fluorine – fluorides – are added to toothpaste to prevent tooth decay.  Bromine is the only liquid non-metal element. It is a reddish-brown colour and evaporates quickly to form a choking, poisonous gas. Bromine is found in seawater and mineral springs in the form of salts, called bromides. Bromine compounds are used in photography, as mild sedatives, and in the manufacture of flameproof coatings and dyes. SILVER BROMIDE IN X-RAYS In X-ray photography, a plastic film is coated with a paste of a bromine compound called silver bromide. When X-ray light strikes the film, the silver bromide breaks apart and pure silver atoms are left on the film. The more intense the light, the more silver atoms are formed and the darker that part of the image becomes. NOBLE GASES Group 18 of the periodic table contains the noble gases. These six unreactive gases do not combine with other elements, so they are usually found on their own. Nearly 1 per cent of air is argon. Traces of neon, helium, krypton, radon, and xenon are also found in air. Argon is used in light bulbs, xenon is used in lighthouse arc lamps, and helium is used to fill airships and hot-air balloons. NEON LIGHTING A neon light is a tube containing a noble gas, but not always neon. When electricity is passed through the tube, the atoms of the noble gas emit (give out) light of different colours. Helium emits a yellow light, neon a red light, argon a blue light, and krypton a purple light. Other colours are created by giving the glass tube different coloured coatings. 15. HYDROGEN You cannot see, taste, or smell hydrogen, yet this element makes up over 90 per cent of matter. The Sun and stars are made of hydrogen gas. On Earth, hydrogen forms compounds (mixture of elements), and is found in almost every living thing. Hydrogen gas is used to make chemicals such as ammonia, which is needed to make fertilizers. Hydrogen is also used to increase the amount of petrol produced from crude oil. HYDROGEN IN STARS Stars are fuelled by hydrogen. At incredibly high temperatures inside stars, hydrogen atoms smash into one another and fuse (join) together to create helium atoms. These reactions give out a huge amount of energy as light and heat. Hydrogen atoms were probably the first atoms to form in the Universe and fuse together to create other, heavier atoms. SPACE SHUTTLE The space shuttle uses liquid hydrogen fuel because hydrogen gives out a lot of power for very little weight. Hydrogen, like all fuel, needs oxygen to burn, so the shuttle has a tank of liquid hydrogen and a tank of liquid oxygen. A fine mist of the two liquids is sprayed into the engines and ignited (set alight). The hydrogen explodes, sending steam out of the nozzles and helping to thrust the shuttle into space. HYDROGENATION Margarine is made by passing bubbles of hydrogen gas through hot vegetable oil. Extra hydrogen atoms bond (join) with the oil molecules, and the oil changes from a liquid to a more solid form. This process is called hydrogenation. If oil is fully hydrogenated, it becomes completely solid; by stopping part way, it becomes a semi-solid. HYDROGEN-FUELLED CAR Scientists are developing hydrogen-powered cars. The cars contain tanks of hydrogen that combine with oxygen from the air to drive them. Hydrogen-fuelled cars produce water instead of polluting exhaust gases. They are not mass-produced, because scientists have not developed a compact and lightweight method for storing hydrogen yet. BIOGRAPHY: ANTOINE LAVOISIER French, 1743-1794 This chemist is often known as the father of modern chemistry. He studied the “inflammable air” that was discovered by English scientist Henry Cavendish (1731–1810). Lavoisier discovered that this gas combines with oxygen to make water. He named the gas hydrogen, which is Greek for water-former.
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ Last edited by Sureshlasi; Sunday, August 03, 2008 at 02:24 PM. |
| The Following User Says Thank You to Sureshlasi For This Useful Post: | ||
Artemis (Sunday, August 03, 2008) | ||
|
#3
|
||||
|
||||
|
16. OXYGEN
On Earth, oxygen is more common than any other element. It is an invisible, odourless gas that makes up 21 per cent of air. Oxygen is found in water, minerals, and almost all living things. It is essential to life. Ordinary oxygen molecules contain two oxygen atoms. Ozone, a three-atom form, is found high up in the atmosphere. Oxygen moves through the environment via the OXYGEN CYCLE. OXYGEN FOR LIFE Divers wear a SCUBA (self-contained underwater breathing apparatus), so they can breathe under water. The SCUBA contains a cylinder of compressed air, which divers carry on their backs. The air is compressed (or squeezed) into the cylinder to increase the amount of air the divers can carry. Divers breathe through a regulator, which decompresses the air as it comes out of the cylinder. BIOGRAPHY: JOSEPH PRIESTLEY British, 1733-1804 In 1774, this chemist announced his discovery of oxygen. He didn’t realize that Swedish chemist Carl Scheele (1742–1786) had found it first, a year or two previously. They both showed that air is not one element. Priestley also discovered how to combine carbon dioxide with water to make fizzy water. OXYGEN CYCLE Almost all living things, including humans, need oxygen to survive. Both plants and animals take in oxygen from their surroundings to release energy. Underwater plants and animals cannot use the oxygen in air – instead they use oxygen dissolved in water. The oxygen cycle continuously circulates oxygen through the environment, so it is always available to all living things. CHANGING OXYGEN Plants are able to use the energy of sunlight to convert carbon dioxide (CO2) and water (H2O) into carbohydrates and oxygen (O2) in a process called photosynthesis. This oxygen is taken in by plants and animals to provide energy, releasing carbon dioxide and water. This process is called respiration. 17. WATER The simple combination of two hydrogen atoms and one oxygen atom creates a water molecule (H2O). Water is the most common compound on Earth, making up over half the weight of living things. It is vital to life, bringing nutrients to and taking away waste from every living cell. Water molecules are attracted to one another through HYDROGEN BONDS, and this gives water some unusual but useful properties. ABUNDANCE OF WATER Water covers around 70 per cent of the Earth’s surface. This is why Earth looks blue from space, and why it is often called the Blue Planet. Water is liquid in the oceans and forms solid ice caps at the ice caps. Water vapour is a gas in air. Humid places, such as rainforests, have a lot of water vapour. The human body is about 60 per cent water and a ripe tomato contains over 95 per cent water. DRINKING WATER At room temperature, pure water is a colourless liquid with a neutral pH – it is not an acid or a base. But most water is not pure. Hard water contains calcium and magnesium minerals, which have dissolved in the water as it flows over rocks. Soap does not lather well in hard water – the minerals react with the soap to form a scum. Hard water is softened by boiling or by passing it through a water softener. HYDROGEN BOND Water molecules have an attraction to other water molecules. This attraction is called the hydrogen bond. It is a fairly weak bond compared to the bonds within a water molecule, but it is still strong enough to give water some unusual properties. For example, water is a liquid at room temperature; other molecules of a similar size are gases. It is also less dense as a solid than as a liquid. MOLECULAR STRUCTURE In a water molecule, electrons are pulled closer to the oxygen atom than the hydrogen atoms. So the oxygen atom has a small negative charge, and the hydrogen atoms have a small positive charge. The slightly positively charged hydrogen atoms of one water molecule are attracted to the slightly negatively charged oxygen atoms of another water molecule. This attraction is the hydrogen bond. 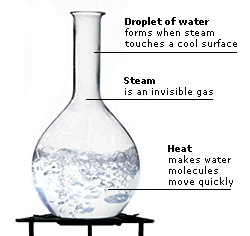 Water boils at 100ºC (212ºF). This is almost 200ºC (424ºF) higher than the boiling points of other similar-sized molecules, such as hydrogen sulphide. Water’s high boiling point can be explained by its hydrogen bonds. Extra heat is needed to break the hydrogen bonds, so a water molecule can break free of other water molecules and leave the liquid’s surface as steam, which is a gas. 18. NITROGEN Nitrogen is needed to make proteins, which are vital to life. Plants and animals recycle nitrogen through the air and soil in a process called the NITROGEN CYCLE. As a gas, nitrogen makes up 78 per cent of air. At everyday temperatures it is very unreactive. It is used in place of air in crisp packets, for example, so the contents do not go stale. Nitrogen is also used to make industrial chemicals such as fertilizers and explosives. LIQUID NITROGEN When nitrogen gas is cooled to -196ºC (-320ºF), it turns to a liquid. Liquid nitrogen is so cold that it can freeze a substance in seconds. In hospitals, it is used to preserve blood and body parts for transplant. The material to be preserved is placed in a special, sealed container that is filled with liquid nitrogen. Because nitrogen is so unreactive, it does not alter the preserved materials in any way. LIGHTNING The heat produced by lightning forces nitrogen molecules in the air to split. Nitrogen atoms bond with oxygen to form nitrogen oxides, which dissolve in water to create nitric acid. Weak nitric acid falls to the soil, where it splits apart to form the compounds nitrates and nitrites. These compounds are essential to life for plants and micro-organisms. FERTILIZING SOIL Farmers often use fertilizers to help their crops grow well. Many fertilizers contain nitrogen in the form of nitrates, because this is the form that plants can use. Natural fertilizers are made from compost and manure. Synthetic fertilizers are made by combining nitrogen from the air with hydrogen from natural gas. EXPLOSIVES Nitrogen compounds are used to make explosives. These compounds contain chemicals that break apart easily to release huge volumes of gases extremely quickly. They can be used in a controlled way to demolish a building without harming other buildings nearby. The explosive TNT (trinitrotoluene) releases hydrogen, carbon monoxide, and nitrogen, and carbon powder, which produces black smoke. NITROGEN CYCLE All living things need nitrogen, but most cannot use nitrogen gas directly from the air. The nitrogen has to be fixed (combined) with other elements to form nitrites and nitrates. This is done by lightning and by nitrogen-fixing bacteria. The nitrates are taken up by plants, which are eaten by animals. This starts the continual cycle of nitrogen called the nitrogen cycle. MOVEMENT OF ATOMS Nitrogen from the air is fixed to make nitrates in the soil by nitrifying bacteria. The nitrates are taken up by plants to build plant protein. When an animal eats a plant, it turns the plant protein into animal protein. Denitrifying bacteria convert the nitrogen contained in animal waste and in decaying plant and animal material back into nitrogen gas again. NITRIFYING BACTERIA IN ROOT NODULES Nitrifying bacteria are a key part of the nitrogen cycle. Some live in the root nodules of legumes (peas and beans), like this nodule from the root of a pea plant. Others live free in the soil. Bacteria in the soil make nitrates from nitrites and other nitrogen molecules. Bacteria in legume root nodules take up nitrates from the soil. 19. CARBON Carbon is the sixth most common element in the universe and is the main element in every living thing on Earth. Carbon atoms are passed between living things through the CARBON CYCLE. Carbon is present as carbon dioxide in the air, and makes up a large part of coal, crude oil, and natural gas. Pure carbon is very rare in nature, although it can be found in one of several different forms, or ALLOTROPES. CARBON AS FUEL Anything that burns well usually contains carbon. Coal, charcoal, wood, and paper are packed full of carbon. Carbon atoms joined together store a lot of energy. When carbon burns, each carbon atom breaks away from its surrounding atoms and reacts with oxygen in the air to form carbon dioxide. The stored energy is released as heat. ALLOTROPE The atoms of some elements can link up in different ways to create different forms called allotropes. Carbon is found in three allotropes: diamond, graphite, and fullerene. Each allotrope has very different physical properties. Graphite, diamond, and fullerene contain only carbon atoms, but the atoms are arranged differently in each allotrope. 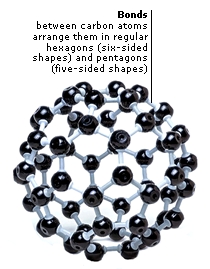 In a fullerene, the carbon atoms link together to form a ball-shaped cage. Fullerenes may contain 100, 80, or 60 carbon atoms. This fullerene contains 80 atoms. The first fullerene discovered was buckminsterfullerene in the 1980s, which has 60 carbon atoms. It is named after Buckminster Fuller, an American architect who designed buildings similar in shape to the fullerene molecule. GRAPHITE Some lubricating engine oils and all pencil leads contain graphite. Graphite has layers of carbon atoms that can slide across one another. There are strong bonds between the carbon atoms of each layer, but weak bonds between the different layers. Because the layers can move over one another, graphite is quite a soft material. GEODESIC DOME The stable structure of fullerenes works well on large-scale buildings. In the 1940s, architect Buckminster Fuller designed a type of building called a geodesic dome. It is made of a network of triangles that together form a sphere. This shape is very stable and encloses a lot of space with little building material, making it strong but light. CARBON CYCLE Carbon atoms continually circulate through the air, animals, plants, and the soil. This recycling of carbon atoms in nature is called the carbon cycle. The bodies of all living things contain carbon. The carbon comes originally from carbon dioxide gas in the air. Green plants and some bacteria take in the carbon dioxide and use it to make food. When animals eat plants, they take in some of the carbon. Carbon dioxide goes back into the air when living things breathe out, and when they produce waste, die, and decay. MOVEMENT OF ATOMS Green plants use carbon dioxide from the air to make food. When an animal eats a plant, it uses the carbon to build body tissue. When the animal breathes out, it returns carbon into the air as carbon dioxide. When the animal dies and decays, the carbon in its body returns to the soil. Decomposers such as worms, bacteria, and fungi, feed on the decaying remains of animals. As they feed, the decomposers breathe out carbon dioxide into the air. Green plants then take in carbon dioxide from the air, and the cycle is repeated. 20. BIOCHEMISTRY The study of the chemical processes of all living things is called biochemistry. These processes include respiration (breathing) and the digestion of food. Carbon atoms can combine in so many ways that living things are mainly made up of molecules containing carbon. The molecule DNA carries the chemical instructions that allow living things to create and make copies of their molecules and reproduce. FOOD FOR ENERGY All living things need food for the energy to make all the other body processes happen, such as growth, movement, and repair. These complicated chemical reactions are called metabolism in animals. Plants make their food, through a process called photosynthesis. BREAKING DOWN CARBOHYDRATES Many foods, including apples, contain molecules called carbohydrates. When broken down through the process of digestion, carbohydrates release a lot of energy. Food contains two other kinds of molecule: fats and proteins. Fats are another good source of energy, and proteins are important for growth. DIGESTION The carbohydrate sugar contains 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. Once eaten, the molecules are broken down to form simpler glucose molecules through the chemical process of digestion. Digestive enzymes, such as amylase, speed up this process. RESPIRATION Glucose molecules pass into the bloodstream and are carried to cells around the body. Every cell uses glucose molecules in a chemical process called respiration. In respiration, the bonds within the glucose molecule break, releasing the energy in the molecule in a form our bodies can use. ENERGY RELEASED Glucose molecules react with oxygen in the air we breathe to release energy and create carbon dioxide (CO2) and water (H2O) molecules. Processes that release energy, such as respiration, are called catabolic reactions. Other processes that take in energy, such as building proteins, are called anabolic reactions. FOOD FOR BUILDING MOLECULES Living things do not only break down molecules, but they also build up complex molecules, such as muscle proteins. Protein molecules are needed for growth. They are made from amino acids, which come from protein-rich food, such as beans, pulses, and meat. AMINO ACID BUILDING BLOCKS Amino acid molecules are made of carbon, hydrogen, oxygen, and nitrogen atoms. They join up to form protein molecules, the building blocks of our bodies. Our 20 different amino acids make thousands of proteins. PROTEIN CELLS The cells of our skin, blood, hair, and muscles are all made up of proteins. There are thousands of different proteins in our bodies, including the enzymes that drive every reaction and the antibodies that fight disease. DNA Probably the most amazing molecule in our bodies is one called deoxyribonucleic acid, or DNA for short. This molecule contains the genes (instructions) for making every different type of protein in our bodies. Almost every cell in our bodies contains DNA, divided up into 46 parts called chromosomes. Each cell uses just the part of the instructions it needs. For example, only a muscle cell makes muscle proteins. MUSCLES FOR MOVEMENT Almost anything we do, such as gymnastics, talking, or reading, relies on our protein-built muscles. Inside every muscle cell, a chemical reaction converts the energy contained within the chemical bonds of ATP (adenosine triphosphate) molecules into movement. This reaction also creates heat, which is why you get hot when you exercise. When ATP molecules are made to store energy, anabolic reactions occur. When energy is released, catabolic reactions occur. 21. ORGANIC CHEMISTRY The study of all compounds that contain carbon is called organic chemistry. Carbon atoms are unique. They can combine with each other to make molecules that contain hundreds, even thousands, of carbon atoms. There are more CARBON COMPOUNDS than compounds of all the other elements put together. CARBON TECHNOLOGY uses carbon compounds to make many modern materials, from the interiors of aircraft to medicines. CARBON IN ALL LIVING THINGS From butterfly wings to the petals of a flower, all living things are made of carbon compounds. All the processes that happen in living things – such as digestion, movement, and growth – are chemical reactions involving carbon compounds. It is the ability of carbon to make so many different compounds that results in the rich diversity of life on Earth. CARBON COMPOUNDS Many carbon compounds contain the same few elements, but in different quantities and arranged in different ways. The most important elements to join with carbon are hydrogen, oxygen, and nitrogen. Carbon atoms can form chains of just carbon and hydrogen, which are called hydrocarbons. They can also form rings of carbon, called aromatics. 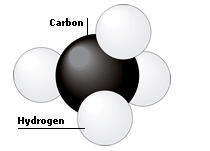 Methane is a hydrocarbon. It contains one carbon atom bonded to four hydrogen atoms. The prefix “meth-” always refers to compounds whose molecules contain only one carbon atom. Methane is a natural gas. It is used in domestic central heating. ALCOHOLS AND ESTERS A carbon compound called an ester gives an apple its distinctive smell. Esters are liquids with a sweet, fruity smell, and evaporate quickly. They are made when alcohol reacts with an acid. Alcohols and esters contain carbon, hydrogen, and oxygen atoms. CARBON TECHNOLOGY The carbon industry is one of the largest and most important industries because so many products contain organic (carbon) compounds. Carbon technology is vital to the production of medicines, paints, synthetic fabrics, food flavourings, plastics, cosmetics, and glues. The raw materials that are the basis for these products come from coal, crude oil, and natural gas.  Racing bikes are often made from carbon fibre because it is strong and light, and can be moulded into complex shapes. The carbon fibres are woven into a cloth which is then cut and layered in a mould. The moulded part is filled with a chemical called a resin and then baked in an oven to form the hard, tough carbon fibre material. CARBON FIBRES Polyacrylonitrile (PAN) is heated to 3,000°C (5,432°F) to create thin filaments of carbon fibre. This material is fireproof and five times lighter than steel, yet twice as strong. Carbon fibre has many uses, such as in lightweight sports equipment, car body panels, construction pipes, and on the wings and nose of space shuttles. 22. PLASTICS Plastics are used to make a wide range of materials, including furniture, computers, and toys. Plastics are not found in nature, but are created from the products of coal, oil, and natural gas. They are made up of carbon, hydrogen, and other atoms linked in long-chained molecules called POLYMERS. Plastics are so useful because they are strong, light and can withstand heat and chemicals better than many materials. They can also be moulded into practically any shape or size.  INSULATING PLASTIC Modern plastics can be created with precise properties that suit a particular use. Mylar® is a plastic used to insulate space shuttles. It is a shiny, strong, and light polyester film made in very thin sheets. It reflects the intense heat generated when a space shuttle re-enters Earth’s atmosphere, protecting the craft and its crew. INJECTION MOULDING Plastic bowls are often shaped by injection moulding. Plastic pellets are heated until they have melted. The liquid plastic is then pushed, or injected, into a mould, held in place by a clamp. After the plastic in the mould has cooled and hardened, the finished product is pushed out. This process is used to mass-produce items such as bowls, butter tubs, and yoghurt pots. COMMON PLASTICS PVC Polyvinylchloride, or PVC for short, is the plastic used to make credit cards and waterproof clothes. It is tough, flexible, cheap to produce, and easy to print on. POLYTHENE There are two types of polythene. Low-density polythene is used to make lightweight plastic bags. High-density polythene is stronger and is used to make plastic milk bottles. PS Polystyrene can be either rigid or foamed. Rigid polystyrene is used to make toys and containers. Foamed polystyrene is used for fast food packaging. PET Polyethylene terephthalate, more commonly called PET, is a strong plastic used to make fizzy drinks bottles. It can be recycled into carpets and ribbons for video cassettes. PP Polypropylene, or PP, is a plastic with a relatively high melting point of 160°C (320°F). It is used to make camera film and dishwasher-proof plastic objects. PA Polyamide, or PA for short, is the plastic used to package oily food such as cheese and meats. It is also known as nylon, and is used in clothing, ropes, carpets, and bristles for brushes. POLYMERS All plastics are synthetic polymers. Polymers are substances whose molecules are made of simpler molecules called monomers joined together in long, winding chains. The monomers contain carbon and hydrogen, and sometimes other elements such as oxygen and nitrogen. Synthetic polymers can be divided into two groups, thermoplastics and thermosets.
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ Last edited by Sureshlasi; Sunday, August 03, 2008 at 02:49 PM. |
| The Following User Says Thank You to Sureshlasi For This Useful Post: | ||
Faryal Shah (Monday, August 04, 2008) | ||
|
#4
|
||||
|
||||
|
GLASS
First made over 5,000 years ago, glass is a thick liquid that never completely sets (hardens). That is why old window panes are thicker at the bottom than at the top. Glass is still in widespread use because it is transparent (see-through), strong, and can be melted and recycled endlessly. Molten glass can be shaped in many ways, including flat panels for windows and threads for optic fibres. GLASS TECHNOLOGY is so advanced that glass can be made fire-resistant and shatterproof. COLOURING GLASS Coloured glass is created by dissolving metal compounds into melted sand. Different metal compounds create different colours. For example, selenium sulphide makes glass red. Iron and chromium compounds produce a deep green glass. MOLTEN GLASS Sand, broken glass, soda, and limestone are heated in a furnace. At around 1,500°C (2,732°F), the mixture melts to form molten (liquid) glass, which is cut into individual globules of glass called gobs. SHAPING The gobs are dropped into bottle moulds. Compressed air blows the glass against the mould walls. The bottles are removed from the moulds and reheated slightly to remove imperfections. COOLING The bottles are cooled slowly on a moving conveyor belt under carefully controlled conditions. This ensures that no dust is trapped in them, and that the glass does not shatter. GLASS TECHNOLOGY Material scientists have developed and improved the properties of glass to suit a range of uses. Heat-proof oven doors are made by adding chemicals to molten glass so that the glass lets light but not heat through. Car windscreens are made shatterproof by cooling molten glass rapidly with jets of air. Test tubes and other glass apparatus used in science labs need to withstand the heat of a Bunsen flame. This kind of glass is made heat-proof by adding boron oxide to the raw materials to make borosilicate. OPTICAL FIBRES Molten glass can be pulled into extremely thin tubes called optical fibres. A beam of light is reflected down the tube, even as it bends around corners. Optical fibres are used in telephone cables. Pulses of light pass down the tube, and transmit information. Optical fibres are also used in endoscopes that allow doctors to see right inside our bodies. CERAMICS The word ceramic comes from an ancient Greek word for “burned earth”. Ceramics are made by firing (heating) clay (fine particles of earth) in an oven called a kiln or furnace. China, bricks, and tiles are made from ceramics. Over the past few decades, ADVANCED CERAMICS have been developed with superior or additional properties to traditional ceramics. PORCELAIN Porcelain has the finest texture of all ceramics. It is made from a white clay called kaolin, fired at very high temperatures. Most ceramics let water through until they are glazed, but porcelain is naturally water-resistant already. It is valued for its glassy smoothness and translucency. THROWING Ceramics can be thrown (made) by shaping a lump of wet clay on a wheel (a turning plate). The potter places the clay on the centre of the wheel, then skilfully raises it into shape by hand. FIRING The pot is fired in a kiln. The first firing, called the bisque firing, hardens the clay. A coating called a glaze is painted onto the pot, and the pot is fired again. Glaze waterproofs the pot. DECORATING Once glazed, a decal (relief) is pre-soaked and smoothed onto the pot. The pot is fired again to stick the decal to the pot permanently. Pots can also be painted with enamel and then fired. BIOGRAPHY: JOSIAH WEDGWOOD English, 1730-1795 This master potter and industrialist introduced many kinds of coloured pottery. He is best known for his Jasper Ware, with classical designs in white on blue or green. He also invented a pyrometer to measure kiln temperature. ADVANCED CERAMICS Bio-ceramics now replace teeth and bones. They are one example of advanced ceramics. Each type is made from a particular component of pure clay. It is heated at a specific temperature, sometimes in a specific gas environment, such as nitrogen. This changes the ceramic’s chemical structure and properties. SPACE SHUTTLE CERAMICS One type of advanced ceramic, called shuttle ceramic tiles, has been created to withstand temperatures up to 1,280°C (2,336°F). Space shuttles are covered in 30,000 of these lightweight tiles. They protect the shuttle from heat when it re-enters Earth’s atmosphere from space. COMPOSITES A composite is a combination of two or more different materials. The new material combines the best properties, such as strength and lightness, of each of the individual materials. There are examples of composites all around us. Boats, bikes, tennis rackets, even dental fillings are all made of composites. Most composites are synthetic materials, but they also occur in nature. NATURAL COMPOSITE Bone is a composite of hydroxyapatite and a protein called collagen. The hydroxyapatite is a brittle but hard and rigid material that gives bone its structural strength. The collagen is soft and spongy, giving bone its flexibility. Bone is 80–90 per cent hydroxyapatite and 10–20 per cent collagen protein. SMART CAR The Smart car is a modern two-seater car that is very light, so it uses less fuel than heavier cars. It has also been designed to take up less room in city centres. Over 40 per cent of a Smart car is made from composite materials. It has been put together in panels, so each composite panel can be easily replaced. RECYCLING Many things we normally throw away can be recycled (used again), including paper, glass, metals, plastics, and BIODEGRADABLE waste such as vegetable peelings and cut grass. Recycling saves natural resources, such as trees and crude oil. It can also save energy as it often takes less energy to make a product from recycled materials than it does to make the product from new materials. For example, 93 per cent more energy is needed to extract aluminium from ore than to recycle it. LANDFILL SITES Most rubbish is dumped in big pits called landfill sites. The pits are lined with clay to prevent poisons leaking into the surrounding soil and polluting water supplies. Pipes are inserted into the pit to collect and remove poisonous methane gas. Unless we recycle more, we will run out of places to put landfill sites. ROLLING STEEL Scrap steel is flattened or shredded, then melted in a furnace. The molten steel is poured into moulds to make slabs of steel called billets. Once solidified, the billets are reheated and rolled into thin sheets. SHEET STEEL Steel sheets are used to make a range of products, such as food cans and car parts. Steel is 100 per cent recyclable. This means that recycled steel is exactly the same as the steel in the original material. RECYCLING PAPER Before recycling, paper is sorted into different grades. It is then mashed with water and chemicals to form a pulp. The pulp is cleaned (to remove staples, glue, or ink) and sprayed onto flat screens. When dry, the paper is used to make new products, such as newspapers. RECYCLED PLASTIC Plastics such as polyethylene terephthalate (PET), used in fizzy drinks bottles, can be recycled. This is because they are a kind of plastic called a thermoplastic. When heated, the plastic melts and can be moulded into a new shape. Only thermoplastics are recyclable. Thermosetting plastics burn rather than melt when heated.  BIODEGRADABLE Materials from living things are usually biodegradable. They break down into simpler substances, often with the help of micro-organisms. Leaves biodegrade into compost and carbon dioxide, both of which recycle in our environment. Most plastics are not biodegradable. They are so different from natural materials that micro-organisms cannot digest them. GARDEN COMPOST Making compost is a good way of recycling biodegradable materials that you would otherwise throw away. Vegetable peelings, sawdust, and grass cuttings can all be layered in a large container. Over a few months, micro-organisms will break down the biodegradable waste into compost. This rich, dark material can be scattered over the soil to provide plants with extra nutrients. (`'•.¸(`'•.¸ ¸.•'´) ¸.•'´) «`'•.¸.¤THE END¤.¸.•'´» (¸.•'´(¸.•'´ `'•.¸)`' •.¸) Chapter no 1
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ Last edited by Shooting Star; Tuesday, May 15, 2012 at 12:10 AM. |
|
#5
|
||||
|
||||
|
Topic # 2
FORCES AND ENERGY 1. FORCES From the movements of the planets to the energy produced inside atoms, everything that happens in the Universe is ultimately caused by forces. A force is a push or pull that can make an object move or TURN around. The bigger the force, the more movement it can produce. When two or more forces act together on an object, their effects are COMBINED. Sometimes the forces add together to make a larger force, and sometimes they cancel each other out. NEWTONS Forces are measured in units called newtons (N), named after English scientist Sir Isaac Newton. The size of a force can be measured using a device called a force meter or newtonmeter. As the load pulls on the hook, it stretches a spring to give a reading on the scale. On Earth, the force of gravity on 1 kg (2.2 lb) is 9.8 newtons. TURNING FORCES If an object is fixed at one point and can rotate around it, that point is called a pivot. If a force acts on the object, the object turns around the pivot. The turning force is called a torque and the effect it produces is called a moment. The bigger the force, the greater the moment. The moment also increases if the force acts at a greater distance from the pivot.  A wheelbarrow is free to pivot around the large wheel at the front. When the worker lifts the handles, the force causes the entire wheelbarrow to swing upwards and turn around the wheel. The long body and handles of a wheelbarrow increase the turning effect and make it easier to tip out a heavy load. INCREASING MOMENTS It is easier to unscrew a nut with a spanner than with your fingers, because the spanner’s long handle increases the turning effect or moment of the force. The size of a moment is equal to the force used times the distance from the pivot on which it acts. If you use a spanner twice as long, you double the moment, and the nut is twice as easy to turn. COMBINED FORCES When forces act in the same direction, they combine to make a bigger force. When they act in opposite directions, they can cancel one another out. If the forces acting on an object balance, the object does not move, but may change shape. If the forces combine to make an overall force in one direction, the object moves in that direction. SUPPORTING A BRIDGE A suspension bridge has to support the weight of its own deck, plus the weight of the vehicles that go across it. The deck of the bridge hangs from huge steel cables suspended over giant pillars. The cables and pillars are arranged so that there is no overall force in any direction. A bridge stays up because the forces on it are balanced and cancel one another out. 2. DYNAMICS Dynamics is the study of how objects move when forces act on them. Normally objects stay still or move along at a steady pace. They resist changes in their motion because of their INERTIA. Once they start moving, they tend to carry on doing so because of their MOMENTUM. Most types of everyday movement can be explained by just three simple LAWS OF MOTION. These were originally worked out by English physicist Sir Isaac Newton. LAWS OF MOTION Newton’s three laws of motion (often called Newton’s laws) explain how forces make objects move. When the forces that are acting on an object are balanced, there is no change in the way it moves. When the forces are unbalanced, there is an overall force in one direction. This changes the object’s speed or the direction in which it is moving. Physicists call a change in speed or direction an acceleration. NEWTON’S 1ST LAW An object will stay still or move along at a steady pace unless a force acts on it. For example, a rocket on a launchpad remains in place because there is no force acting on it to make it move. NEWTON’S 2ND LAW When a force acts on an object, it makes the object change speed or move in a different direction. When the rocket’s engines fire, the force they produce lifts the rocket up off the launchpad and into the air. NEWTON’S 3RD LAW When a force acts on an object, the object pulls or pushes back. This reaction is equal to the original force but in the opposite direction. As the hot gases shoot down from the engines, an equal force pushes the rocket up. BIOGRAPHY: SIR ISAAC NEWTON English, 1642–1727 Newton’s three laws of motion enabled him to produce a complete theory of gravity, the force that dominates our Universe, and to explain why the Moon circles round Earth. Newton also made major discoveries about optics (the theory of light) and explained how white light is composed of many colours. INERTIA Newton’s first law explains that objects remain where they are or move along at a steady speed unless a force acts on them. This idea is known as inertia. The greater the weight (or mass) of an object, the more inertia it has. Heavy objects are harder to move than light ones because they have more inertia. Inertia also makes it harder to stop heavy things once they are moving. CRASH-TEST DUMMIES As a car accelerates, passengers are thrown backwards; when a car brakes or crashes, passengers are thrown forwards. In both cases, this is because the inertia caused by their mass resists the change in movement. During crash-tests, dummies that weigh the same as a human body are used to help test safety belts and airbags. MOMENTUM Moving objects carry on moving because they have momentum. The momentum of a moving object increases with its mass and its speed. The heavier the object and the faster it is moving, the greater its momentum and the harder it is to stop. If a truck and a car are travelling at the same speed, it takes more force to stop the truck because its greater mass gives it more momentum. COMPARING MOMENTUM A foal is smaller and has less mass than a horse. When a foal and a horse gallop along together at the same speed, the horse has more momentum because of its greater mass. This means that it is easier for the foal to start moving, stop moving, and change direction than the horse. The momentum of a moving object is equal to its mass times its velocity. 3. FRICTION If you kick a ball across a playground, it bounces and rolls on the ground’s rough surface and soon comes to a halt. What slows it down is friction, which is the force between a moving object and whatever it touches. Cars travel faster if they are STREAMLINED to reduce a type of friction called air resistance. Friction can sometimes be helpful. Without friction between the tyres and the road, cars would not have enough grip to go around corners. LUBRICATING MACHINERY Slippery substances such as oil reduce the friction between two surfaces. This is known as lubrication. Machinery has to be lubricated to prevent its moving parts from wearing out due to friction. Most machines are oiled or greased when they are made and are lubricated from time to time as they are used. STREAMLINING When objects move, the air around them generates a type of friction called air resistance, or drag, that slows them down. Fast-moving objects such as cars, trains, and aeroplanes are all streamlined – designed with curved and sloping surfaces to cut through the air and reduce drag. This helps them to move faster and use less fuel. Boats can be streamlined too, to reduce water resistance. 4. ELASTICITY Forces make things move, but they can also stretch things, squeeze them, and change their shape. A rubber ball changes shape when you use force to squeeze it, but it returns to its original shape when you stop squeezing. Materials that do this have elasticity. They are made up of particles called molecules that can stretch apart. Other materials, such as modelling clay, change shape easily when a force is applied, but they do not return to their original shape when the force is no longer applied. These materials have PLASTICITY. TRAMPOLINING A trampoline is made of stretchy rubber fastened to a metal frame by metal springs. When you land on a trampoline, you stretch the rubber and the springs. Both rubber and springs are elastic. As they return to their original shape, they pull back upwards and push you into the air. PLASTICITY Materials have plasticity when they are easily moulded into shape and do not return to their original shape when the moulding force is removed. When we talk about plastics, we usually mean various colourful materials that have been made out of chemicals produced from oil. In fact, the word plastic applies to any material that can be easily moulded into different shapes. Even metals can be plastic because, if heated, they soften and can be shaped. 5. MOTION Everything in the world is moving. Even things that seem still are in motion, because the atoms inside them are vibrating. An object moves from one place to another when forces act on it and those forces are not balanced. When a force in one direction changes the SPEED or VELOCITY of an object, or the way it moves, this is known as ACCELERATION. 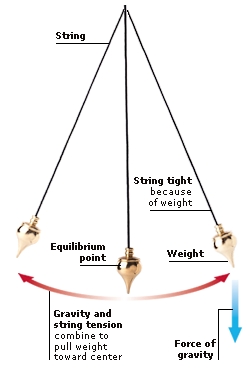 A clock’s pendulum moves back and forth because the forces that act on it are not balanced. The weight on the pendulum and the tightness of the string constantly try to pull the pendulum towards the centre. But its weight and speed swing it past the point of balance (equilibrium point). So the velocity of the pendulum is constantly changing. SPEEDING ROLLER COASTER A roller coaster’s carriages accelerate (gather speed) when the force of gravity pulls them down a steep incline. The speed and weight of the carriages then keeps them moving, even when they continue in a straight line or climb upwards. SPEED When we think of speed, we think of cars, jet planes, anything that moves quickly. To scientists, however, speed means things moving fast or slow. Speed is defined as the distance an object travels in a certain amount of time. Fast cars travel at higher speed than slow cars, so they can go further in the same time. MEASURING SPEED You can calculate the speed of a runner by measuring the time he takes to travel a certain distance. His speed is the distance he travels divided by the time he takes. If the distance is measured in metres and the time in seconds, the speed is measured in metres per second (mps). VELOCITY Velocity is the speed of an object moving in a particular direction. Two cars driving at the same speed have different velocities if one of them goes north and the other goes south. Velocity is measured in metres per second (mps), which divides the distance travelled by the time taken, in a specific direction.  ACCELERATION When we talk of things accelerating, we usually mean they are speeding up. In science, however, acceleration means any change in an object’s velocity, whether it goes faster, slower, or changes direction. According to Newton’s second law of motion, a force is always needed to produce an acceleration. The bigger the force, the faster the change in velocity. 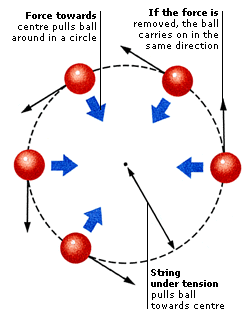 An object that moves in a circle, such as this ball swinging on a string, constantly changes direction. Even when it turns at a steady speed, its velocity is always changing. It takes a force to make it accelerate like this. When an object moves in a circle, the force that constantly pulls it towards the centre and stops it flying off in a straight line is called centripetal force.
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ |
|
#6
|
||||
|
||||
|
6. GRAVITY
Gravity is the force that makes things fall to the ground on Earth and holds the planets in their orbits (paths) around the Sun. The force of gravity acts over immense distances between objects in the Universe and holds them all together. The gravitational force between objects increases with their MASS. It also increases the closer they are. The gravity between objects on Earth is usually too small to notice. CENTRE OF GRAVITY On Earth, objects have a point, often near their centre, which is called their centre of gravity. The lower it is, the more stable they are. Cars are designed with their heavy engines near to the ground, to keep their centre of gravity low. This means they can corner at speed without tipping over. FALLING FORCE Which falls faster, a ball or a feather? In Earth’s atmosphere, the ball reaches the ground first because air resistance slows the feather down. In a vacuum, there is no air and therefore no air resistance. The feather and the pool ball fall at the same rate because gravity pulls them with exactly the same amount of force. MASS The mass of an object is the amount of matter it contains. The greater the mass of an object, the more matter it contains, and the more it pulls on other objects with the force of gravity. The mass of an object does not vary unless the amount of matter inside it changes for some reason. Mass is measured in kilograms (kg). 7. RELATIVITY Einstein realized that the speed of light is always the same. He then calculated that an object travelling near this speed acts strangely: it shrinks in length, increases in mass, and time slows down. He also calculated that mass alters space. So small objects do not travel in straight lines near a large object – instead they follow the distortions in space made by it. Centuries after gravity was identified as a force, Einstein’s theory of relativity explained why it works the way it does.  In traditional physics, gravity attracts one mass to another. This explains why a comet follows a curved path around the Sun. Einstein’s general theory of relativity explains gravity differently. Masses warp space and time a bit like heavy balls resting on a sheet of rubber. The bigger the mass, the more distortion, and the greater the pull of gravity. In 1921 Einstein was proved correct when the light from a star was shown to be bent by the warping effect of the Sun’s mass. ACCURATE TIMEKEEPING The effects of relativity are only detectable when things travel at very high speeds. To detect them, scientists need accurate clocks that use atoms to tell the time. Atoms of the element caesium vibrate at a precise rate. Atomic clocks measure time by counting these vibrations. Clocks such as the one above use a radio link to a central atomic clock to relay the precise time. BIOGRAPHY: ALBERT EINSTEIN German, 1879–1955 When Albert Einstein was expelled from school, no one imagined he would become one of the most brilliant physicists of the 20th century. His theory of relativity was so strange that people refused to believe it at first. It was widely accepted only after he won the Nobel Prize for Physics in 1921. 7. PRESSURE When you press or push something, the force you apply is called pressure. Pressure is measured as the force you use divided by the area over which you use it. If you use a bigger force, or if you use the same force over a smaller area, you increase the pressure. We experience AIR PRESSURE all the time because of the weight of air pressing in on our bodies. WATER PRESSURE increases as you go deeper in the ocean. AIR PRESSURE The gases in Earth’s atmosphere are made up of tiny molecules that are constantly crashing into your body and trying to press it inwards. This pressing force is called air pressure. It is greatest at ground level where there are most air molecules. At greater heights above Earth, there are fewer air molecules and the air pressure is much less. It is possible to compress (squeeze) air, and this is used to inflate vehicle tyres and to power machines such as pneumatic drills. AIR PRESSURE IN TYRES Heavy construction machines have large tyres for two reasons. The compressed air in the tyre helps to absorb bumps, so the ride is much smoother than it would be with a solid wheel. Large tyres also help to spread the weight of the machine over a much bigger area. This reduces the pressure on the ground and stops the machine sinking into the mud. WATER PRESSURE Water behaves differently from air when it is under pressure. It cannot be compressed (squeezed). This makes it useful for transmitting force in machines, using a system called hydraulics. Water is also heavier than air, and an increase in water pressure affects humans more than a drop in air pressure. Even with a snorkel or other breathing apparatus, it feels much harder to breathe underwater. The water above you presses down from all sides on your body, so your lungs find it harder to expand to take in air. The deeper you go, the more water there is above you and the greater the pressure on your body.  Liquid pressure is used to carry force through pipes. The small force pushing down does not compress the liquid but moves through the liquid to push another piston a small distance upwards. The wider area of this piston increases the force applied. CHANGING AIR AND WATER PRESSURE The higher we go, the less air there is in the atmosphere above us. The deeper in the sea we go, the more water there is pressing down on us. 20,000 m (65,600 ft) HIGH At this height, air pressure is less than one-tenth that at sea level. AIRLINERS 11,000 m (36,000 ft) Aircraft cabins are pressurized to allow us to breathe as easily as at sea level. Oxygen is also supplied in case of emergency, as there is less air at this height. MOUNTAIN TOPS 7,500 m (24,600 ft) At this height, climbers often use breathing apparatus to give them more oxygen. SEA LEVEL The human body is ideally adapted to deal with the air pressure at sea level. 120 m (400 ft) DEEP Divers cannot go any deeper than this without special suits to protect them from the pressure of the water. SUBMERSIBLES 6,500 m (21,300 ft) Underwater craft such as submarines have strong, double-skinned hulls to withstand water pressure. The world’s deepest-diving crewed submersible can dive to 6,500 m (21,300 ft). 10,000 m (32,800 ft) DEEP At this depth, the pressure of water is 1,000 times greater than it is at sea level. 8. ENERGY Scientists define energy as the ability to do work. Energy makes things happen. The energy in sunlight makes plants grow, the energy in food enables us to move and helps us to keep warm, and the energy in fuel powers engines. Energy comes in many different forms and can be converted from one form into another. The main types include POTENTIAL ENERGY , KINETIC ENERGY, and CHEMICAL ENERGY. POTENTIAL ENERGY Energy that is stored up ready to be used in the future is called potential energy, because it has the potential (or ability) to do something useful later on. An object usually has potential energy because a force has moved it to a different position or changed it in some other way. When an object releases its stored potential energy, this energy is converted into energy of a different form. ELECTRICAL POTENTIAL ENERGY When thunderclouds move through the sky, they build up a large amount of electricity inside themselves. This is known as static electricity, which is a store of energy. When a cloud builds up more static electricity than it can store, some of the electricity flows from the cloud to Earth in a bolt of lightning. ELASTIC POTENTIAL ENERGY This type of potential energy powers bows and catapults. It takes effort to stretch a piece of elastic or rubber because the forces between its molecules try to resist being pulled apart. As the elastic stretches, the molecules move away from one another and gain potential energy. The energy stored in stretched elastic can also be used to power such things as toy cars and model aeroplanes. GRAVITATIONAL POTENTIAL ENERGY A snowdrift on top of a mountain has a huge amount of potential energy. This is known as gravitational potential energy because it is gravity that is constantly trying to pull the snow down the mountain to the bottom. When an avalanche occurs, the snow gathers speed and its stored potential energy is turned into kinetic energy (the energy of movement). KINETIC ENERGY Moving objects have a type of energy called kinetic energy. The more kinetic energy something has, the faster it moves. When objects slow down, their kinetic energy is converted into another type of energy, such as heat or sound. Objects at rest have no kinetic energy. Kinetic energy is often produced when objects release their potential energy. HAMMER STRIKING NAIL A moving hammer has a lot of kinetic energy. As it strikes the nail, it slows down and loses its kinetic energy. The energy does not disappear, however. Some of it goes to split the wood to make way for the nail, some passes into the wood as heat energy, and some is converted into sound. CHEMICAL ENERGY This is the energy involved in chemical reactions, when elements join together into compounds. This energy is stored inside the compounds as chemical potential energy. The stored energy can be released by further chemical reactions. The food we eat stores energy that is released by digestion. Energy can also be released by burning the chemicals in a process called combustion. Fuels are chemical compounds that release heat energy by combustion.  FOOD AS CHEMICAL ENERGY When humans or other animals eat food, they use its stored energy to keep warm, maintain and repair their bodies, and move about. Different types of food store different amounts of energy. The amount of energy a food contains is measured in kilocalories (called Calories for short). 9. WORK Scientists use the word work to describe the energy needed to do a task, by making a force move through a distance. The amount of work done is equal to the energy used and both are measured in JOULES (J). It takes energy to lift a weight a certain distance, because you have to do work against the force of gravity. POWERFUL machines can do lots of work in a short time. EFFICIENT machines waste relatively little energy when doing work. EFFICIENCY Efficiency is a measure of how much of its energy a machine converts into useful work. No machine ever converts all its energy into work: some energy is always wasted in the process. Car engines convert fuel into the energy they need in order to move, but get hot as they do so. This heat does not help the car to move, so a car is relatively inefficient, compared to other machines. EFFICIENT MACHINE Bicycles are efficient machines. They allow riders to convert muscle power into movement with little wasted energy. Racing cyclists wear aerodynamic clothing. Less energy is wasted overcoming air resistance, so more energy is used to move the bicycle. POWER Some machines can do work more quickly than others, and these are said to be more powerful. Power is the amount of work that something can do in a certain amount of time. Cars with bigger engines can go faster, which means they cover more distance in the same time. This means faster cars do work more quickly than slower cars, so they are more powerful machines. JOULES The amount of work done when a force acts over a distance equals the size of the force (measured in newtons) times the distance through which it moves (measured in metres). The work done is measured in joules, named after English physicist James Prescott Joule (1818–1889). An amount of work takes the same amount of energy to do it, so energy is also measured in joules. ONE JOULE One joule is the work that has to be done to make a force of one newton act over a distance of one metre. One joule of energy is needed to do one joule of work. It would take two joules of work to apply the same force for a distance of two metres. HIGH-ENERGY FOODS When a tennis player hits the ball, he does work. If he eats a banana before the match, his body can use the energy it contains to do this work. The energy value of food is measured in kilojoules or kilocalories (Calories for short). The body does not convert all the energy in food into useful work, so it is not 100% efficient. 10. HEAT Metal heated in a furnace shows that it is hot by glowing red and sending out sparks – but there is also some heat in ice and snow. Heat is the energy of movement, or kinetic energy, stored inside every object, hot and cold alike. Heat energy makes the particles (atoms and molecules) inside the object move about. TEMPERATURE is how hot or cold an object is, depending on its heat energy. Temperature is measured with a THERMOMETER. MOLTEN METAL When iron is heated in a furnace, it glows red-hot and then melts at a temperature of 1,535°C (2,795°F). At this temperature, its particles move about with lots of kinetic energy. At higher temperatures they move even faster, and the iron in the furnace starts bubbling. ICEBERG Ice is cold, but it still contains some heat energy. An iceberg is made up of particles of water, held in a rigid crystal structure. They still vibrate slightly. If the iceberg cooled down so that its particles stopped moving altogether, it would be at the lowest possible temperature that can ever, in theory, be reached. This is absolute zero. TEMPERATURE Temperature is a measure of how hot or cold something is. Things that have high temperature are hotter than things that have lower temperature, because they have more heat energy inside them. Any object can transfer heat energy to a colder object. As it does so, it cools down and its temperature falls. The colder object warms up and its temperature rises. TEMPERATURE SCALES Temperature is measured in degrees Celsius or Fahrenheit (°C or °F) or on the absolute temperature scale, in units called Kelvins (K). The Celsius (also called Centigrade) scale runs from freezing point (0°C) to boiling point (100°C). THERMOMETER This is a device that measures how hot or cold something is on a temperature scale. When things get hotter, their heat energy makes them expand or get bigger. This is how a thermometer measures temperature. As the liquid inside expands, it creeps up a tube, which is marked with a scale and numbers that show the temperature. MERCURY IN BULB The thermometer contains a small amount of liquid mercury in a glass bulb at the bottom. To take someone’s temperature, the glass bulb is placed inside their mouth. As the mercury is warmed by the person’s body, it expands up the tube, and climbs the temperature scale. A kink in the tube stops the mercury falling back too quickly, so the temperature can be read and recorded. THERMOSTAT A thermostat switches an air-conditioning unit on and off to keep a room at a constant temperature. As the room heats up, the brass strip inside the thermostat expands more than the iron strip attached to it. The strip bends inwards, completes an electrical circuit, and switches on the air-conditioning unit.
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ |
|
#7
|
||||
|
||||
|
11. RADIOACTIVITY
The atoms of some chemical elements are unstable. They try to rearrange themselves to make more stable atoms. In the process, they give off radiation particles or tiny bursts of radiation. This process is called radioactivity. Although radioactivity can be harmful to people, it can also be important to us in everyday life. It is used to make nuclear energy and preserve food, and it also plays a vital role in the treatment of cancer. DANGER: RADIATION Some types of radioactivity are harmful, because they damage or destroy the tissues of the human body. If people receive large doses of radioactivity, they can become ill with radiation sickness, which often causes cancer. Radiation sickness can also affect people’s ability to have children. TYPES OF RADIOACTIVITY The three types of radiation are alpha and beta particles, and gamma radiation, named after the Greek letters above. An alpha particle is two protons joined to two neutrons. A beta particle is an electron. Gamma radiation is high-energy electromagnetic radiation. ALPHA DECAY An alpha particle is made when the nucleus (central part) of a large, unstable atom rearranges itself, or decays, to make a smaller, more stable atom. The new and smaller atom has two protons and two neutrons fewer than the original atom. These join together to make the alpha particle that is given off. Some energy is also released as a gamma ray. This is high-energy and high-frequency radiation, travelling at the speed of light. 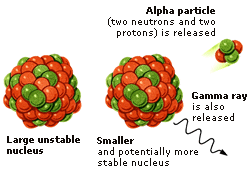 BETA DECAY Beta decay is quite different from alpha decay. One of the neutrons in the nucleus of the unstable atom changes into a proton and an electron. The proton joins onto the nucleus, but the electron is ejected from the atom at high speed. This fast-moving electron is called a beta particle. Some energy is also released as a gamma ray. 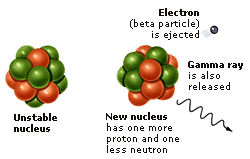 12. NUCLEAR ENERGY Atoms are small but can release lots of energy. When an unstable atom changes into a more stable one, it gives off radioactivity. It also gives off some of the potential energy locked inside the nucleus of the atom. Some atoms can be made to produce a constant supply of nuclear energy in a process called a chain reaction. Nuclear energy makes possible the destructive power of nuclear bombs, but it also generates much of the world’s electricity. NUCLEAR EXPLOSION When a nuclear bomb detonates, it starts a runaway chain reaction and releases enormous amounts of energy very quickly. A lump of radioactive plutonium the size of a tennis ball can produce as much energy as tens of thousands of tons of powerful explosives. NUCLEAR FISSION In nuclear fission (splitting), large atoms break into smaller ones and give off energy. When a neutron is fired at the nucleus of a large atom, the atom becomes unstable and splits into two smaller atoms. Energy is produced and some neutrons are given off too. They collide with more large unstable nuclei of the original material and continue the chain reaction.  NUCLEAR FUSION In nuclear fusion (joining), massive energy is given off when small atoms fuse together to make larger atoms. A neutron is released at the same time. Stars like the Sun make their energy when nuclear fusion happens inside them at extremely high temperatures and pressures. Scientists are hoping that nuclear power stations will one day use fusion to provide Earth with a clean and inexpensive source of energy. BIOGRAPHY: LISE MEITNER Austro-Swedish, 1878-1968 Physicist Lise Meitner was one of the first to explain the process of nuclear fission. She also predicted the idea of the nuclear chain reaction before anyone had managed to make it work. She supported development of nuclear power, but opposed the production of nuclear bombs. 13. ENERGY SOURCES Everything we do takes energy, which we get from many different sources. Most of the energy on Earth originally came as light and heat from the Sun. It has been stored in fuels such as coal and oil, formed from the fossilized remains of plants or animals over millions of years. Supplies of these fossil fuels are limited. This is why we are now turning to supplies of RENEWABLE ENERGY that never run out. Another alternative is GEOTHERMAL ENERGY, produced deep inside the Earth.  This power station is burning coal to release the chemical energy it contains and produce electricity. The Sun’s energy is converted into carbon-based compounds by plants as they grow. When plants decay, they change into a dark, earthlike substance called peat. Over millions of years, the peat is buried under other material and pressure turns it into coal. ENERGY FROM THE SUN Every second, the same amount of energy reaches Earth from the Sun as a coal-fired power station could make from about 200,000 truckloads of coal. The Sun makes energy from nuclear reactions deep inside it. In some ways it is like a giant nuclear power station. INSIDE A NUCLEAR POWER STATION Nuclear reactions take place in the fission reactor of a nuclear power station. Coolant (cold water) is pumped around the reactor and is turned to steam by the heat generated by the reactions. The steam drives an electricity-generating machine called a turbine. 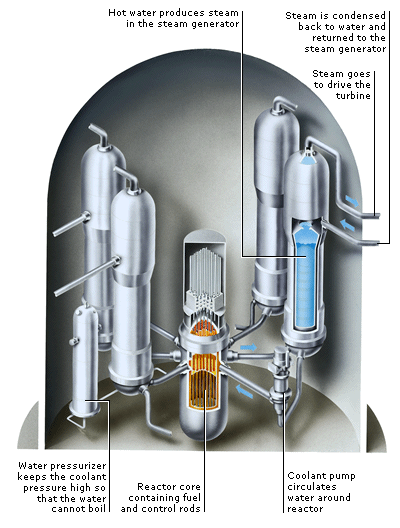 RENEWABLE ENERGY Long after fossil fuels have run out, the tides will still be turning, the wind will still be blowing, and the Sun will still be shining. Ocean, wind, and solar power is called renewable energy because it never runs out. Using renewable energy is better for the environment. Unlike fossil fuels, it produces no harmful pollution and does not add to the problem of global warming. 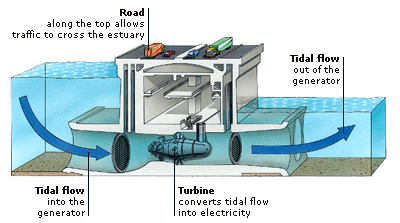 As the tides ebb and flow, they make water move back and forth in rivers that end in estuaries at the coast. A tidal power generator is a type of bridge that blocks the mouth of an estuary so the tide has to move through it. Each time the water flows in or out, it turns a turbine inside the power generator and produces electricity. GEOTHERMAL ENERGY This form of energy is not generated by the Sun. It is made by the nuclear reactions taking place all the time deep inside the Earth. These make heat energy in the Earth’s core, and the heat moves around inside the Earth by convection. Volcanoes and hot geysers release geothermal energy at the Earth’s surface. GEOTHERMAL PLANT A geothermal energy plant takes its power from the Earth’s heat. It works by pumping cold water down a hole drilled into the Earth. The Earth’s geothermal energy heats up the water and it returns to the surface as hot water and steam. The hot water can be pumped to homes and factories nearby. The steam is used to drive a turbine and make electricity. 13. MACHINES In science, a machine is any device that changes a force into a bigger or smaller force, or alters the direction in which a force acts. Machines come in all shapes and sizes. Large machines such as cranes, bulldozers, and tipper trucks are based on smaller, simpler machines called LEVERS , WHEELS , PULLEYS , SCREWS, and GEARS. Simple tools such as a spade, a knife, a drawing-pin, and a nutcracker are also machines. LEVERS Most levers are force multipliers. They reduce the effort needed to work against a force called the load. They magnify a small force into a larger force. When a force acts on an object that is fixed at one point, the object turns around this pivot point. The further away the force is from the pivot point, the easier it is to turn the object. That is how levers make work easier. TYPES OF LEVERS Levers can work in three ways. Class one and class two levers turn the effort into a larger force to work against the load. Class three levers work in the opposite way, to reduce the force and increase the control of it over a greater distance. WHEELS A wheel and the axle it turns around combine as a machine that works like a lever. The distance between the rim of the wheel and the axle multiplies either speed and distance or force. If the effort is applied to the axle, the rim of the wheel turns further, and so faster, than the axle, but with less force. If the effort is applied to the rim of the wheel, the axle turns with more force but not so far or fast. 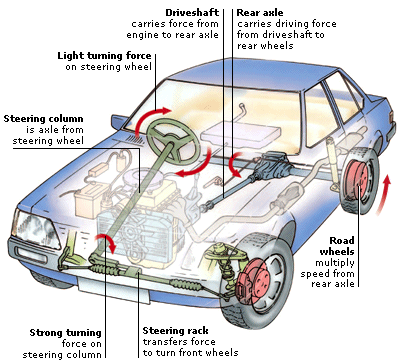 A steering wheel multiplies force because the rim turns further than the steering column. It multiplies your effort and turns the car’s wheels with more force than you actually apply. The road wheels multiply speed. The car’s engine turns the driveshaft and rear axle at a certain speed. The axle turns the large road wheels further and therefore faster. 14. ENGINES Many modern machines, from motorbikes to jet aircraft, are powered by engines. An engine is a machine that turns fuel into movement. The fuel is burned to generate heat energy. The heat is then converted into mechanical power. In a car or motorbike engine, the power comes from pistons and cylinders. In a jet aircraft, power comes from hot gases rushing past a spinning wheel called a TURBINE. Engines also produce various waste gases, which cause pollution. TURBINES These are machines that extract and use the energy from a moving liquid or gas. Windmills and waterwheels were the very first turbines. Their sails and paddles took power from the movement of wind and water. Turbines are still important today, especially as they are used in power stations and jet engines. TURBOJET ENGINE An aeroplane’s jet engine has one or more large fans at the front. These mix air with fuel and compress the mixture. In the combustion chamber, the fuel ignites, burns, and produces hot gases. As the gases expand, they turn a turbine that spins the fans. The force of the hot gases rushing backwards out of the engine propels the aircraft forwards. 15. FLOATING When an object such as a boat or an airship rests in a fluid (a liquid or gas), it has to displace (push aside) some of the fluid to make room for itself. The object’s weight pulls it downwards. But the pressure of the fluid all around the object tries to push it upwards with a force called upthrust. The object SINKS if the upthrust is less than its weight, but floats if the upthrust is equal to, or more than its weight. FLOATING AIRSHIP Hot air is less dense than cooler air, so the hot air in a balloon weighs less than the same volume of cool air. The weight of the airship pulls it downwards, but the air around the balloon pushes it upwards with a force called upthrust. If the upthrust is equal to or greater than the total weight of the balloon, the basket, and the hot air, the airship floats. FLOATING SHIP When a boat floats, it displaces some of the water underneath it. As the weight of the boat pushes down on the water, the water pushes up on the boat with an upward force called buoyancy. The larger the boat, the greater the buoyancy. The boat floats if the buoyancy is as great as or greater than the weight of the boat. BIOGRAPHY: ARCHIMEDES Greek, 287-212 BC Archimedes is best known for realizing that a floating object displaces its own weight in a fluid. Legend has it that he worked this out in his bath. As he stepped into the bathtub, water splashed over the side. He found that the weight of this water equalled his body weight. This idea is known as Archimedes’ Principle. SINKING Not everything will float. A block of wood will float on water, but a lump of iron exactly the same size will sink. This is because a piece of wood of a certain size weighs less than the same volume of water, so wood floats on water. However, iron is much heavier than either wood or water. A block of iron weighs more than the same volume of water. This is why iron sinks in water. PLIMSOLL LINE The more cargo a ship carries, the deeper it sits in the water. Ships also displace varying amounts of water according to the saltiness and temperature of the water. This varies from ocean to ocean around the world. Large ships have a mark called a Plimsoll line painted on their sides. This shows how much weight they can safely carry in different parts of the world. 16. ENERGY WAVES Many different kinds of energy travel in waves. Sound waves carry noises through the air to our ears. SEISMIC WAVES travel inside the Earth and cause earthquakes. Light, heat, radio, and similar types of energy are carried by a variety of waves in the ELECTROMAGNETIC SPECTRUM. Some energy waves need a medium, such as water or air, through which to travel. The medium moves back and forth as waves carry energy through it, but it does not actually travel along with the wave. 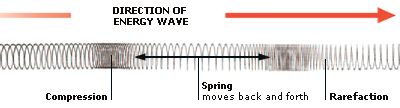 Suppose you fix a slinky spring at one end and push the other end back and forth. Some parts of the spring, called compressions, are squeezed together. Other parts of the spring, called rarefactions, are stretched out. The compressions and rarefactions travel down the spring carrying energy. This type of wave is called a longitudinal or compression wave. 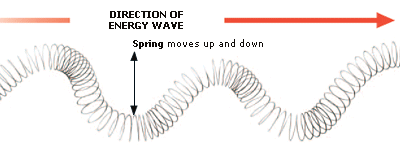 Suppose you fix the slinky at one end and move it up and down. Energy travels along the spring’s length in S-shaped waves, so the forward direction in which the energy moves is at right angles (or transverse) to the up-and-down direction of the movement of the spring. This is called a transverse wave. OCEAN WAVES When an ocean wave crashes against the shore, it releases a large amount of energy. Ocean waves are transverse waves that carry huge amounts of energy across the surface of the sea as they move up and down. A wave 3 m (10ft) high carries enough energy to power around 1,000 lightbulbs in every 1 m (3 ft) of its length. BIOGRAPHY: HEINRICH HERTZ German, 1857-1894 In 1887, physicist Heinrich Hertz became the first person to prove that waves carry electromagnetic energy between two places. This extremely important finding eventually led to the development of radio and television. Hertz did not live to see these inventions, however. He died in 1894, aged only 36. ELECTROMAGNETIC SPECTRUM Radios, televisions, mobile phones, and radar use signals made up of electromagnetic waves. These are waves that carry energy as electricity and magnetism at the speed of light. Light we can see is also an electromagnetic wave, but other types of electromagnetic wave are invisible. The various types of electromagnetic wave have different frequencies and wavelengths. Together, they make up the electromagnetic spectrum. GAMMA RAYS These are produced by radioactivity. They have a short wavelength and a high frequency and carry large amounts of energy. They are very harmful and can cause cancer in humans and animals. ULTRAVIOLET RAYS These invisible waves are slightly shorter than visible violet light and carry more energy. We wear sunglasses and sunblock to prevent damage to our eyes and skin by ultraviolet rays. INFRARED RAYS Infrared rays are slightly longer waves than visible red light. Although we cannot see infrared, we can feel it as heat. When heat energy is transferred by radiation, it is carried by waves of infrared. RADAR Radar is a way of locating aeroplanes and ships using a type of radio waves called microwaves. These have much longer wavelengths than visible light. Cooking is another use for microwaves. RADIO WAVES Radio waves are the longest in the spectrum. They carry radio and TV signals around Earth. Radio waves from outer space are picked up by radio telescopes and used in studies of the universe. SEISMIC WAVES When the energy stored in rock deep inside the Earth is suddenly released, it travels up to the Earth’s surface in huge seismic shock waves. The waves move along weaknesses in the rock known as faults. As they do so, they produce violent shaking of the ground and an earthquake. The largest earthquakes can release as much energy as a small atomic bomb. 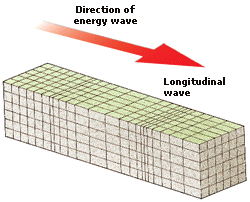 Some seismic waves are longitudinal or compression waves called primary or P-waves. They cause damage by pushing and pulling things back and forth in the same direction that the wave travels. P-waves move extremely quickly through the Earth’s interior at a speed of about 25,000 kph (15,500 mph). EARTHQUAKE Earthquakes kill around 10,000 people every year worldwide. Many cities now have buildings that absorb the energy in seismic waves. They may wobble, but they do not collapse.
__________________
ஜ иστнιπg ιš ιмթΘรรιвlε тσ α ωιℓℓιиg нєαят ஜ |
| The Following 3 Users Say Thank You to Sureshlasi For This Useful Post: | ||
ASP imran khan (Monday, August 16, 2010), Na_shah (Thursday, April 16, 2009), Qaiserks (Wednesday, April 01, 2009) | ||
 |
«
Previous Thread
|
Next Thread
»
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Principles of Political Science | Xeric | Political Science | 8 | Friday, December 02, 2011 12:19 AM |
| Science and Muslim Scientists | Wounded Healer | Islamic History & Culture | 0 | Wednesday, May 09, 2007 06:21 PM |
| Philosophy of Science | A Rehman Pal | Philosophy | 0 | Sunday, March 18, 2007 03:42 PM |
| Science Terminology | ummera | General Knowledge, Quizzes, IQ Tests | 0 | Sunday, October 22, 2006 09:57 PM |
| Barriers to Science Journalism in Pakistan | Qurratulain | Journalism & Mass Communication | 0 | Saturday, April 22, 2006 01:44 AM |













