Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy.
Like all catalysts, enzymes take part in the reaction - that is how they provide an alternative reaction pathway. But they do not undergo permanent changes and so remain unchanged at the end of the reaction. They can only alter the rate of reaction, not the position of the equilibrium.
Most chemical catalysts catalyse a wide range of reactions. They are not usually very selective. In contrast enzymes are usually highly selective, catalysing specific reactions only. This specificity is due to the shapes of the enzyme molecules.
Many enzymes consist of a protein and a non-protein (called the
cofactor). The proteins in enzymes are usually globular. The intra- and intermolecular bonds that hold proteins in their secondary and tertiary structures are disrupted by changes in temperature and pH. This affects shapes and so the catalytic activity of an enzyme is pH and temperature sensitive.
Cofactors may be:
- organic groups that are permanently bound to the enzyme (prosthetic groups)
- cations - positively charged metal ions (activators), which temporarily bind to the active site of the enzyme, giving an intense positive charge to the enzyme's protein
- organic molecules, usually vitamins or made from vitamins (coenzymes), which are not permanently bound to the enzyme molecule, but combine with the enzyme-substrate complex temporarily.
How Enzymes Work
For two molecules to react they must collide with one another. They must collide in the right direction (orientation) and with sufficient energy. Sufficient energy means that between them they have enough energy to overcome the energy barrier to reaction. This is called the
activation energy.
Enzymes have an
active site. This is part of the molecule that has just the right shape and functional groups to bind to one of the reacting molecules. The reacting molecule that binds to the enzyme is called the
substrate.
An enzyme-catalysed reaction takes a different 'route'. The enzyme and substrate form a reaction intermediate. Its formation has a lower activation energy than the reaction between reactants without a catalyst.
A simplified picture
Route A reactant 1 + reactant 2 ----------- product
Route B reactant 1 + enzyme ------------intermediate
intermediate + reactant 2 ---------------- product + enzyme
So the enzyme is used to form a reaction intermediate, but when this reacts with another reactant the enzyme reforms.
Lock and Key Hypothesis
This is the simplest model to represent how an enzyme works. The substrate simply fits into the active site to form a reaction intermediate.
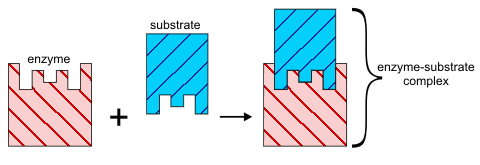 Induced fit hypothesis
Induced fit hypothesis
In this model the enzyme molecule changes shape as the substrate molecules gets close. The change in shape is 'induced' by the approaching substrate molecule. This more sophisticated model relies on the fact that molecules are flexible because single covalent bonds are free to rotate.
Factors affecting catalytic activity of enzymes
Temperature
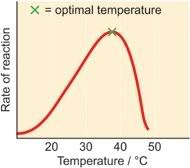
As the temperature rises, reacting molecules have more and more kinetic energy. This increases the chances of a successful collision and so the rate increases. There is a certain temperature at which an enzyme's catalytic activity is at its greatest (see graph). This optimal temperature is usually around human body temperature (37.5 oC) for the enzymes in human cells.
Above this temperature the enzyme structure begins to break down (
denature) since at higher temperatures intra- and intermolecular bonds are broken as the enzyme molecules gain even more kinetic energy.
ph
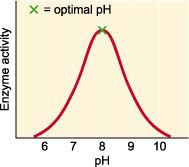
Each enzyme works within quite a small pH range. There is a pH at which its activity is greatest (the optimal pH). This is because changes in pH can make and break intra- and intermolecular bonds, changing the shape of the enzyme and, therefore, its effectiveness.
he rate of an enzyme-catalysed reaction depends on the concentrations of enzyme and substrate. As the concentration of either is increased the rate of reaction increases (see graphs). For a given enzyme concentration, the rate of reaction increases with increasing substrate concentration up to a point, above which any further increase in substrate concentration produces no significant change in reaction rate. This is because the active sites of the enzyme molecules at any given moment are virtually saturated with substrate. The enzyme/substrate complex has to dissociate before the active sites are free to accommodate more substrate.
Provided that the substrate concentration is high and that temperature and pH are kept constant, the rate of reaction is proportional to the enzyme concentration.
Inhibition of enzyme activity
Some substances reduce or even stop the catalytic activity of enzymes in biochemical reactions. They block or distort the active site. These chemicals are called
inhibitors, because they inhibit reaction.
Inhibitors that occupy the active site and prevent a substrate molecule from binding to the enzyme are said to be
active site-directed (or
competitive, as they 'compete' with the substrate for the active site).
Inhibitors that attach to other parts of the enzyme molecule, perhaps distorting its shape, are said to be
non-active site-directed (or
non competitive).
Immobilized enzymes
Enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Pectinase is used to produce and clarify fruit juices. Problems using enzymes commercially include:
- they are water soluble which makes them hard to recover
- some products can inhibit the enzyme activity (feedback inhibition)
Enzymes can be immobilized by fixing them to a solid surface. This has a number of commercial advantages:
- the enzyme is easily removed
- the enzyme can be packed into columns and used over a long period
- speedy separation of products reduces feedback inhibition
- thermal stability is increased allowing higher temperatures to be used
- higher operating temperatures increase rate of reaction
There are four principal methods of immobilization currently in use:
- covalent bonding to a solid support
- adsorption onto an insoluble substance
- entrapment within a gel
- encapsulation behind a selectively permeable membrane