Atomic And Nucleur Physics
Structure of an Atom
An Atom consists of two parts
i.Nucleus
ii.Extra Nucleur space
i.
Nucleus
Nucleus occupies only a small fraction of the total volume of an atom and is at the centre of an atom. The two main particles of nucleus are the positively charge proton and neutron with no charge. The mass of both is almost equal and is 1.67 x 10-27 kg.
 ii.Extra Nucleur Space
ii.Extra Nucleur Space
In this space negatively charged particlle (electron)moves around the nucleus in orbits of various shape. The mass of an electron is 9.1 x 10-31 kg
ATOMIC NUMBER
The number of protons in the nucleus of an atom is called atomic number. It is denoted
'Z'. It is also called charged number.
MASS NUMBER
The total number of protons and electrons in the nucleus of an atom is called mass number. It is denoted by
'A'
The no's of nuetron
"N" present in nucleus is given by.
If the atomic number of an atom "X" is A and atomic mass number is Z then this atom is represented by the symbol "
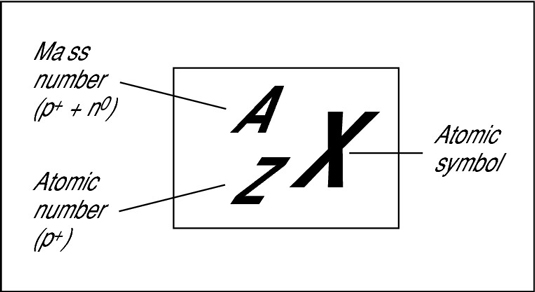
wch is called the nucllide.
For example there is only one proton in the nucleus of H atom so its atomic no is 1 and atomic mass number is also 1 .
ISOTOPES
Isotopes are those elements whose atomic numbers are same but their mass number are different.
For Example Hydrogen has three aisotopes represented by

Quote:
|
Isotopes are neutral atom contains same no of protons and electrons
|
DIFFERENCE BETWEEN ATOMIC NUMBER AND ATOMIC MASS NUMBER
TOMIC NUMBER- It is the number of protons in the nucleus of an atom.
- It is also called cahrge number.
- It is identity of an atom.No two elemenets can have same atomic number.
- It is denoted by "Z".
- It is equal to numbers of orbiting electron in a neutral atom.
- It is always less than atomic mass number for an element.
ATOMIC MASS NUMBER- It is the sum of the numbers of proton and neutron in a nucleus.
- It is also termed as mass number.
- Different isotopes of two elements may have same atomic mass number.
- It is denoted by "A".
- The numbers of neutrons is equal to or greater than the number of protons in a nucleus
- Atomic mass number is alwasy greater than the atomic number of an element
NATURAL RADIOACTIVITY
Radioactivity is such a process in which the elements with the charge number(atomic number)greater than 82 naturally keep on radiating.
Radioactive Element
Element which have charge number greater than 82 are unstable and they emit radiations continuously. Such types pf elements are called radioactive elements.
Characteristics- The atom of radioactive elements change into atoms of the other elements due to emission of radiations.
- Radioactivity is a spontaneous process and contineous for a long time.
- The process of radioactivity is irreeversible.
Radioactivity was discovered by Henry Becqueral in 1986. He found that an ore containing Uranium emits an invisible radiation that penetrates through a black paper wrapping a photographich plate and affects the plate. In order to test his conclusion ;he performed many experiments.
Bacqueral took a closed box into which no light could enter and placed in it the uranium salt and photographic plates near to each other and also seprated by Aluminium sheets. In both the cases he obtained the same results. This shows that uranium salt emits strong radiation and they are not affected by the absence of presence of the sunlight.
Artificial Radioactive Elemenets
Using modern techniques, the elements whih are much lighter than the natural radioactive elements are made radioactive. Such elements are called artificial rdiactive elements.
Thus we include that all the radioactive from the radioactive material are not alike these are of 3 types , namely Alpha, Beta and Gamma radiations.
(α)Alpha Particles
The radiations that bend towards the negative plate of magnetic field and are composed of positively charged particles called α particles.
Properties- The speed of α ranges between 1.4x10 7 m/s and 1.7 x 10 7 m/s
- α-particles ionizes the gas through which they pass.
- α-particles affect the photographic plate.
- They produces fluorescence in zinc sulphide.
- At atmospheric pressure they travel small distance in air.
- α-rays are positively charged particles because they carry a charge twice that of proton
- The mass of α particle is four times of helim atom
- In fact α -particles are also called helium nuclei.
- Each α-particle carries a positive charge of +3.20 x 10-19 C
- In Strong electric or magnetic field they show the same deflections as positive carges does.
β(beta)-Particles
The radiations that bend towards the positive plate of magnetic field are composed of negative charged particles called β-particles.
Properties- The speed of β-rays ranges from 1 to 99 % of speed of light.
- β-particles produces ionization in air but this is less than α-particles.
- β-particles also affect the photographic plate.
- They produces fluorescence in barrium platinocynide.
- They can pass through the thick layer of matter.
- β-particles are also affected by electric and magnetic fields
- β-particles are also called fast moving electrons.
- They arry negative charge.
- The mass of β-particles is equeal as that of electron
- They can cause scintillation in certain substances like anthracene.
Gamma Particles
Thse radiations that go straight without bending means that having no charges are called gama particles.
Properies
- They moves with the speed of light
- The ionization produced in a gas y them is very small as compared to alpha particles.
- Gamma rays affect the photographic plates but this effect is much more than Beta rays.
- Gamma rays produces fluorscence in barrium platinocynide.
- The penetrating power of gamma rays is much more than beta particles.
- Gamma rays are such electromagnetic waves which are emitted by the nucleus
- They are simillar to x rays but they have hort wavelength.
- They have diffraction and interference phenomenon.
- They are not affected by electric and magnetic field.
- They can cause scintillation in certain substance like sodium iodide and zinc sulphide ZnS
HALF LIFE
The time during which the number f atoms of an element are reduced to one half is known as half life of an element.
We know that radioactive elements continuously emit radiations due to which they changes into new elements.
- If an α-particles is emitted by an element then.
- If an β particle is emitted by an element
The atomic no of the newly produced element remains the same but its charge number increases by one unit.It is due to fact that protons and neutrons are present inside the necleus which continuously change into one another. When a neutron emits a β particles ,it changes into proton.So the charge of the new element is increased by one unit.
Parent and Daughter Element
During radiation emission reaction, the original element is called the parent element and newly formed element is called the daughter element.The number of breaking atoms is proportional to the no of atoms left in that element.this means that no of initially breaking atoms is large which decreases with time.
NUCLEAR FISSION REACTION
The breaking of nucleus into two parts wih the release of large amount of energy is called nuclear amount of energy is called nuclear fission reaction
Types of Nuclear Fission Reactions
There are two types of reactions
1.Controlled Fission Reaction
2. UnControlled Fission Reaction
Controlled Fission Reaction
A system in which the fission reaction is controlled is called a controoled fission reaction.
If the fission reaction is controlled then the desired amount of energy can be produced in a useful manner.In the nuclear reactor. Boron or Cadmium rods absords the sur neutrons and thus nuclear fission reaction can be controlled.
UnControlled Fission Reaction
In the chain reaction as the fission reaction builds up the liberat energy goes on increasing. Th fission reaction thus become uncontrollable and hence whole of the matter explodes which resluts in great destruction. Such a reaction is used in atomic bomb.
NUCLEAR FUSSION REACTION
AProcess in which the light nuclei diffuse to form a heavier nucleus is called fusion reaction
During the fusion reaction mass is converted in to energy, which is subsequently released mainly as heat and other forms of energy. Th energy output of such fusion process is even larger than that released during nuclear fission reaction.
Difficult to produce Fussion reaction.
It is very difficlut to produce a fussion reaction because when nuclei are brought near each other for fusion , work has to against the elctrostatix force, which requires amount of energy or heat.
Source of solar energy
Scientist considered that the energy commimg from the sun and the stars is due to fussion nucleons.For producing alpha particles hydrogen has to be converted into helium.
Hydrogen Bomb
is an example of fussion reaction.For fussion reaction the required temprature is achieved by exploding an atomic bomb.
uDIFFERENCE BETWEEN FISSION AND FUSSION REACTIONS
FISSION REACTION- In this process heavy nucleus splits up into light nuclei.
- Energy is needed to start fission reaction.
- In fission reaction unit mass produces small energy than fusion reaction
- Fission reaction is controllable
FUSSION REACTION- In this process light nuclei fuse togatherto produce nucleus.
- A very high temprature is needed to start fission reaction.
- In fussion reaction unit mass produces largeenergy than fision reaction
- Fussion reaction is uncontrollable
Continue
Kindly correct me if sime error is done.