FEDERAL PUBLIC SERVICE COMMISSION
COMPETITIVE EXAMINATION FOR
RECRUITMENT TO POSTS IN BS-17
UNDER THE FEDERAL GOVERNMENT, 2015
CHEMISTRY - PAPER I
TIME ALLOWED: THREE HOURS
PART - I (MCQS): MAXIMUM 30 MINUTES
PART - I (MCQS): MAXIMUM MARKS = 20
PART - II: MAXIMUM MARKS = 80
NOTE: (i) Part - II is to be attempted on the separate Answer Book.
(ii) Attempt ONLY FOUR questions from PART - II. ALL questions carry EQUAL marks.
(iii) All the parts (if any) of each Question must be attempted at one place instead of at different places.
(iv) Candidate must write Q. No. in the Answer Book in accordance with Q. No. in the Q.Paper.
(v) No Page/Space be left blank between the answers. All the blank pages of Answer Book must be crossed.
(vi) Extra attempt of any question or any part of the attempted question will not be considered.
(vii) Use of Calculator is allowed.
PART - II
Q. No. 2. (a) What is Pauliís Exclusion Principle? (5)
(b) Give the electronic configuration of Sodium. (5)
(c) What are Bohrís postulates and how do they explain the hydrogen atom
spectrum? (10) (20)
Q. No. 3. (a) Define Heat of combustion. How is it experimentally determined? (10)
(b) For the combustion of 1 mole of benzene at 25įC, the heat of reaction at constant pressure is given by (10) (20)
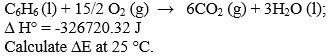
Q. No. 4. (a) Describe the manufacture of Phosphorus on a large scale. (5)
(b) Draw the figure of Nitrogen cycle in nature. (5)
(c) Compare the physical properties of three allotropic forms of Carbon. (10) (20)
Q. No. 5. (a) How is steel manufactured? Describe various chemical reactions taking place in the blast furnace. (10)
(b) Describe the electrolytic refining of Copper. (10) (20)
Q. No. 6. (a) What are fertilizers? Why are they needed? Describe various types of fertilizers and their uses. (10)
(b) Describe the processes of Urea manufacturing in Pakistan. (10) (20)
Q. No. 7.(a) What are transition metals? Discuss their characteristic features. (5)
(b) Draw molecular orbital diagrams of
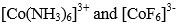
(10)
(c) What was Rutherfordís atomic model? (5) (20)
Q. No. 8. (a) What is Greenhouse Effect? How does it cause global warming
of Earth? (10)
(b) What is meant by water pollution? Discuss various sources of water
pollution. (10) (20)
***************